RESEARCH ARTICLE
Systematic Review and Meta-Analysis of Health-Related Quality of Life in Patients with β-Thalassemia that Underwent Hematopoietic Stem Cell Transplantation
Olga Mulas1, *, Ilaria Pili1, Marco Sanna1, Giorgio La Nasa1
Article Information
Identifiers and Pagination:
Year: 2023Volume: 19
Issue: Suppl-1, M3
E-location ID: e174501792301031
Publisher ID: e174501792301031
DOI: 10.2174/17450179-v17-e211208-2021-HT2-1910-4
Article History:
Received Date: 16/02/2021Revision Received Date: 13/09/2021
Acceptance Date: 10/11/2021
Electronic publication date: 05/01/2023
Collection year: 2023

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
β-Thalassemia major (β-TM) represents one of the most important hemoglobinopathies worldwide. Remarkable improvements have been achieved in supportive therapy based on blood transfusions and iron chelation, and nowadays, this approach is capable of assuring a long life in these patients in industrialized countries. The only curative treatment is represented by hematopoietic stem cell transplantation (HSCT). However, this treatment may be burdened by deterioration in the Health-Related Quality of Life (HRQoL). This paper aimed to evaluate the role of HRQoL in transplanted β-TM patients with a systematic review and meta-analysis.
Methods:
PubMed database, Web of Science, and Scopus were systematically searched for studies published between January 1st, 2000 to September 2020. The following terms were entered in the database queries: β-thalassemia, HRQoL, and HSCT. The study was carried out according to the Preferred Reporting Items for Systematic and Meta-analyses (PRISMA) statement.
Results:
We identified a total of 33 potential studies. Among these, 10 were finally considered in the systematic review and 5 in the meta-analysis. Overall, good scores in the principal domains of HRQoL were reported by transplanted patients. These data were confirmed by results of meta-analysis that showed significant difference between transplanted and β-TM patients treated with conventional therapy in the physical and emotional dimension, with a medium effect size [d=0.65, 95% CI (0.29-1.02), z = 3.52, p =0.0004, I2=75%; and d=0.59, 95% CI (0.43-0.76), z = 6.99, p <0.00001, I2=0%, respectively].
Conclusion:
HRQoL is generally good in β-TM transplanted patients and may significantly contribute in deciding whether or not to transplant a β-TM patient treated with conventional therapy.
1. BACKGROUND
Beta-thalassemia major (β-TM) is among the most common genetic diseases that affect millions of children throughout the world [1]. Over time, β-TM has been the protagonist of countless advances both in terms of understanding the molecular and pathogenic mechanisms that determine it, as well as in its treatment. Indeed, patients born in the last decades can have a life expectancy comparable to healthy individuals with standard therapy based on transfusion support and iron-chelation [2]. However, many differences remain between industrialized and developing countries, where the availability of economic resources for public health is still insufficient. The maintenance of a high standard of treatment requires a massive expenditure of resources that heavily affect health spending. This means that, especially in developing countries, where the disease occurs at a very high frequency, an adequate level of care cannot always be offered to the patients [3]. Hematopoietic stem cell transplantation (HSCT) is currently the only definitive cure for β-TM. It is the only one capable of determining definitive healing in a large number of patients, especially in those children and young adults who arrive at the transplant procedure regularly transfused and those who underwent a good iron-chelation regime [4]. Unfortunately, HSCT procedure can be burdened by short-term side effects (rejection, acute graft versus host disease - GVHD) and long-term side effects (chronic GVHD, major organ dysfunction, increased incidence of solid tumors), without a negligible risk of mortality [5-7]. A good outcome of HSCT is also related to the immunogenetic status of donors and recipients [8]. The final therapeutic choice is difficult and requires a careful evaluation of several aspects.
Health-related quality of life (HRQoL) is generally conceptualized as a multidimensional construct referring to patients’ perception of the impact of disease and treatment on their physical, psychological, and social functioning and well-being [9]. However, studies that have evaluated this aspect in the transplanted β-TM are very limited.
The aim of this paper was to perform a systematic review and meta-analysis of the main studies that have addressed the HRQoL in β-TM patients who underwent HSCT in comparison with conventional therapies, other diseases, or HRQoL of the general population, in order to assess HRQoL benefits of the transplant procedure.
2. MATERIALS AND METHODS
2.1. Search Strategy
A systematic literature search on PubMed, Web of Sciences, and Scopus was performed to find studies on HRQoL in transplant β-TM patients. The studies were published from January 1st, 2000 to September 30th, 2020. Using MeSH headings, we searched for the terms “bone marrow transplantation”, “stem cells transplantation”, “allogeneic”, “quality of life”, “HRQoL”, “Health-related quality of life,” “thalassemia”,” β-thalassemia”, “thalassemia major” as well as variations thereof. A more extensive description of the search strategy is shown in Table S1. The results were defined using the Preferred Reporting Items for Systematic and Meta-analyses (PRISMA) statement to identify, select, and determine the eligibility of papers for inclusion in the study. The PRISMA 2009 checklist is attached in (Fig. 1) shows the study flow diagram. Quality rating of studies was performed by NIH Study Quality Assessment Tools through different questions that evaluate several domains [10] and the results are shown in Table S2.
2.2. Inclusion and Exclusion Criteria
According to the standard PICOS approach, we defined the following eligibility criteria: our participants (P) are thalassemia patients, the intervention (I) is hematopoietic stem cell transplantation, the comparison (C) group includes non-transplanted thalassemia patients and the general population, and the primary outcome (O) is the quality of life. Studies were included in this study if: (1) they were published in peer-reviewed journals; (2) they were published in the English language; (3) they reported evaluation of HRQoL in β-TM patients that underwent HSCT in comparison with β-TM patients treated with conventional therapy, other transplantable diseases, or the general population; (4) they were longitudinal or cross-section studies.
Studies were excluded if: (1) they were in languages other than English; (2) they were case series, reviews, letters, editorials, or commentaries; (3) they reported an analysis of HRQoL of β-TM patients without distinctions between treatments. Two reviewers (O.M. and I.P.) autonomously identified studies for potential inclusion. After exclusion of duplicates (including articles repeatedly reporting the results of the same trial or with overlapping samples) and articles that were unrelated to the main topic, individual studies were included when they matched the inclusion criteria. Discrepancies between the reviewers were resolved by consulting a third experienced researcher (G.LN.).
2.3. Statistical Analysis
In one study, data of mean and standard deviation was not available [11] and was calculated from median and ranges according to Luo et al. and Wan et al. [12, 13]. Statistical analyses were performed through the t-test on dependent variables. Standard mean difference and a 95% confidence interval (CI) were calculated. To assess heterogeneity between the studies, the chi-squared test (for evaluation of heterogeneity between studies statistically; P less than 0.05) and I2 index (to evaluate the heterogeneity of the results) were used. All meta-analyses were conducted using Review Manager 5.4 and were performed by pooling the standard effect sizes using a random-effects model. This model assumes that within-group variability in scores and mean effect size are caused by differences between studies. The common metric in the study was the standardized mean difference according to Cohen (Cohen’s d), which is an appropriate effect size comparison between two means. Cohen’s d was used to estimate effect size using the following interpretation: small (0.2), medium (0.5), and large (0.8).
The QoL questionnaires utilized were QLQ-C30 [14], the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) [15], WHOQOLBREF (HK) [16, 17], and PedsQL questionnaires [18, 19]. From each measure, several dimensions were considered: physical, emotional, and social function and a more accurate description of each questionnaire can be found in the Table S3. If available, the Pesaro risk classification was evaluated. This classification predicts outcomes of hematopoietic stem cell transplantation for TM patients. Three variables were found to significantly influence transplant outcomes: hepatomegaly (defined in terms of centimetres below the costal arch), liver fibrosis (absent or present), and chelation history (regular or irregular) [20].
3. RESULTS
We initially screened 247 articles; after the removal of duplicates, we recorded 218 articles and kept 33; 10 of the 33 articles were assessed for eligibility (Fig. 1). Twenty-three articles were excluded. Of these, 12 did not evaluate QoL outcomes or bone marrow transplant patients; 2 did not consider β-TM patients or lacked in principal outcomes;7 were reviews, and 2 articles did not clearly distinguish between β-TM patients who underwent transplantation. Finally, 10 studies were included in the qualitative synthesis and 5 in the quantitative. Based on the inclusion and exclusion criteria, 10 articles were included [11, 21-29] for qualitative analysis, with a total sample of 395 patients examined. The total number of patients included in the quantitative analysis was 576. Transplanted β-TM patients were compared with β-TM patients treated with standard therapy such as red cells transfusion and iron chelation therapy. A small number of studies have compared transplanted β-TM patients to the general population or transplanted patients with other hematologic diseases.
Overall, 5 papers were considered for the quantitative analysis between transplanted and conventionally treated β-TM patients [11, 21, 24, 25, 28]. Full characteristics of the studies examined in this systematic review are resumed in Table 1.
3.1. Qualitative Analysis
Javanbakht et al. compared 44 transplanted β-TM patients with 74 transfusion-dependent patients in Iran. The time elapsed between transplant and HRQoL assessment was 8 months and 18 years. Physical and emotional domain scores, evaluated by using SF-36, were statistically higher in transplant patients (physical function: 93.07 vs. 84.93, P=0.004; role limitations due to physical problems: 77.84 vs. 58.88, P=0.003; role limitations due to emotional problems: 78.79 vs. 55.92, P=0.001). The authors concluded that HSCT give better HRQoL compared to standard therapy [21].
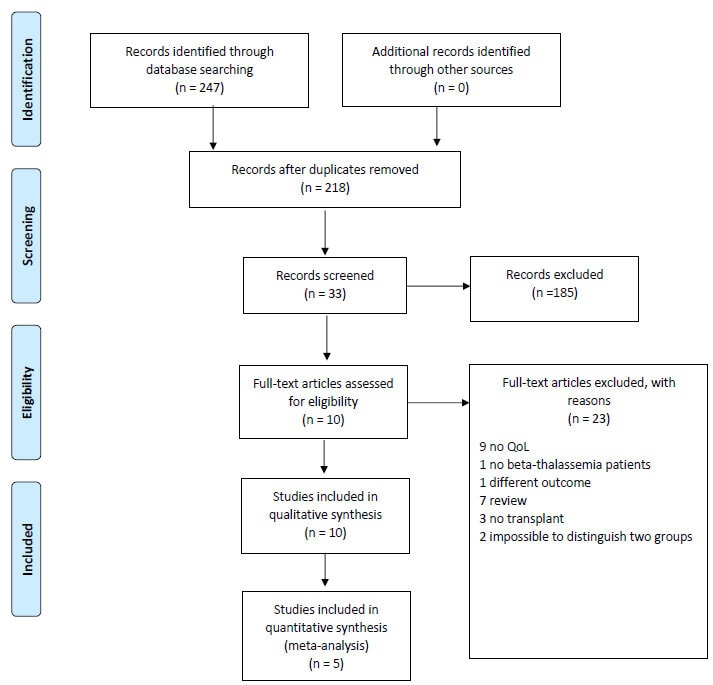 |
Fig. (1). PRISMA 2009 Flow Diagram. |
| Author | Year | N° Patients | Mean Age Years, (Range) of Transplanted Patients | Pesaro Stade TM | Type Questionnaire | Study Design | Comparison Cohort |
| Bahar [27] | 2005 | 84 | 9.5 | ND | KINDL | Cross-sectional | No transplant beta-thalassemia |
| Caocci [22] | 2006 | 19 | 22 (17-37) | III | EORTC QLQ-C30 | Cross-sectional | None |
| Cheuk [28] | 2008 | 24 | 15.2 (5.3-30.4) | I, II, III | WHOQOL-BREF(HK), PedsQL | Cross-sectional | No transplant beta-thalassemia |
| Caocci [11] | 2011 | 28 | 10 (5-17) | II, III | PedsQL | Longitudinal | Same patients before transplant |
| Kelly [23] | 2012 | 6 | 8 (5-18) | ND | CHRIS-General domains | Longitudinal | Transplant for other hematological diseases |
| Uygun [26] | 2012 | 49 | 11.6 (3.5-27.5) | ND | WHOQOL-BREF Peds QoL |
Cross-sectional | No transplant beta-thalassemia |
| La Nasa [24] | 2013 | 109 | 34 (21-48) | I, II, III | SF-36, FACT-BMT | Cross-sectional | No transplant beta-thalassemia and health |
| Javanbakht [21] | 2015 | 44 | 20 (5-35) | ND | SF-36 | Cross-sectional | No transplant beta-thalassemia |
| Caocci [29] | 2016 | 71 | 33 | ND | SF-36 | Cross-sectional | No transplant beta-thalassemia and health |
| Patel [25] | 2018 | 40 | 10 (5-18) | ND | Peds QoL | Cross-sectional | No transplant beta-thalassemia |
Caocci et al. investigated HRQoL in a cross-sectional study of 19 β-TM patients, evaluated by EORTC QLQ-C30. The time elapsed between transplant and HRQoL assessment was 300 days or more. The mean of general HRQoL after unrelated donor HSCT was good (mean score: 76.4). The comparison of patients with or without GVHD showed that the mean of general HRQoL was good (mean score: 65.3) in the first cohort and very good (mean: 81.9) in the second one. No statistical difference was found in global health analysis but patients without GVHD reported better value in physical function (95 vs. 82.2, P=0.04), emotional function (89.6 vs. 66.7, P=0.01), pain (4.2 vs. 33.3, P=0.01), and insomnia (0 vs. 27.8, P=0.02) [22].
La Nasa et al. compared 109 β-TM transplant patients with the general population and with another control cohort of 124 β-TM patients treated conventionally, evaluated by SF-36 and FACT-BMT. The median time elapsed between transplant and HRQoL assessment was 22.8 years (range: 11.7-30.3). The comparison of β-TM HSCT patients and the general population showed lower scores in the general health scale (69.9 vs. 72.2, P=0.005). However, the sub-analysis on GVHD showed higher scores in β-TM transplanted patients without GVHD compared with the general population in mental health (11.1; 95%CI, 3.8-18.3, P=0.003), role emotional functioning (15.9; 95% CI, 4.5-27.3; P=.007), and mental component summary (5.3; 95% CI, 1.6-9, P=0.006). Worse scores were observed in the patients >15-years for general health (-12.8, 95%CI, -23.1 to -2.6, P=0.015) and physical component summary (-4.3, 95%CI, -8.2 to -0.3, P=0.035). In addition, better outcomes were found in transplanted patients compared with transfusion-dependent patients, especially in role limitations due to physical functioning (94.50 vs. 71.45), bodily pain (84.53 vs. 63.29), and role emotional functioning (92.05 vs. 74.37) scales [24].
Caocci et al. collected HRQoL data, by SF-36, on some patients evaluated in the previous paper and compared 71 β-TM transplant patients with their sibling donors and another group of 71 conventionally treated patients. The median time elapsed between transplant and HRQoL assessment was 21 years. Physical health scores of transplanted patients were not statistically different from those of their sibling donors (68.4 vs. 70.4, P = NS) but were significantly higher in comparison with patients under transfusions and iron chelation (68.4 vs. 61.6, P = 0.01) [29].
Cheuk et al. evaluated a transplant β-TM group of 24 patients and a conventional treatment group of 74 patients by different questionnaires. The median time elapsed between transplant and HRQoL assessment was 6.5 years (range: 1.1-13.5 years). In the cohort of patients older than 12 years, the WHOQOL-BREF(HK) questionnaire was administered. Overall health and physical health were better in the transplant group in comparison with the transfusion group (3.67 vs. 3.06, P=0.01 and 75.20 vs. 63.94, P=0.007, respectively). Similarly, the personal relationship domain was better in transplanted patients (4.13 vs. 3.69, P=0.014). Subdivision by the Pesaro score, which evaluates the mortality risk of transplantation in β-TM patients based on hepatomegaly, liver fibrosis, and compliance to iron chelation [20], showed that the class II of risk has a significantly higher score in the psychological domain than class III (72.6 vs. 56.2, P=0.042). No differences were found in psychological, social relationships, and environment domains. In the other cohort (<18 years), the PedsQL questionnaire was used. The overall scores in emotional, social, psychosocial, and school domains were not significantly different between the transplant and non-transplant groups. Scores in the physical domain were similar in the 2 groups, but transplant patients reported better scores in running (3.53 vs. 2.72, P=0.001) and sports exercises (3.2 vs. 2.64, P=0.038) [28].
Uygun et al. split patients by age and showed that higher HRQoL scores were found in the group of 49 patients aged between 5-18 years and those who underwent HSCT, compared to the group of 50 non-transplanted age-matched (77.65 vs. 71.77 P=0.045) patients. Particularly, in 5-7-year-old patients, differences were found in the emotional domain (77.5 vs. 54 P=0.035), while in the 8-12-year-old group, differences were present in the physical and school domains (81.3 vs. 69 P=0.012 and 79.5 vs. 70.7 P=0.046). In the adult group, the physical domain and the overall health showed higher scores in transplanted patients compared to the transfusion group (79.7 vs. 66.6, P=0.041 and 80.5 vs. 60.4 P=0.034, respectively). The presence of GVHD decreased HRQoL in transplanted patients (81.5 vs. 65.5 P=0.030). The median time elapsed between transplant and HRQoL assessment was 4.4 years (range: 2.01-11.96 years). HRQoL was assessed usingWHOQOL-BREF (HK) and PedsQL instruments. Due to these results, the authors concluded that HSCT should be performed before primary school [26].
Patel et al., in a recent paper, showed similar results using PedsQL in two Indian β-TM cohorts. The median time elapsed between transplant and HRQoL assessment was 5 years (range: 2-10 years). In the transfusion group (60 patients), HRQoL scores were lower than in the transplanted cohort (40 patients), especially in physical (83.6 vs. 92.8, P <0.001), emotional (84.4 vs. 93.5, P <0.001), school (74.8 vs. 88.6, P <0.001), and psychosocial domains (82.7 vs. 91.6, <0.001). Significant differences among means of same domains were found when the comparison was made between transplanted children and transfusion-dependent adult patients in physical (73.5 vs. 92.8, P <0.001), emotional (74.5 vs. 93.5, P <0.001), school (67 vs. 88.6, P <0.001), and psychosocial domains (76 vs. 91.6, <0.001) [25].
Bahar et al. found better HRQoL scores in 84 β-TM patients who underwent HSCT compared to 71 non-transplanted patients in psychological (65.1 vs 75.2), social relationship (24.3 vs 24.1), and physical domains (116.7 vs 107.2) [27]. The measurement of HRQoL was assessed in patients using the KINDL scale 6 months after the transplant.
Caocci et al. reported HRQoL longitudinal data in a study where 28 β-TM Middle Eastern patients were evaluated through PedsQL questionnaire, before transplant and at different time points after this procedure, particularly after 3, 6, and 18 months post-transplant. The transplant impacted the general well-being with a reduction of mean scores from baseline to 3 months after HSCT (81 vs. 75.7). A progressive increase was observed at 6 months post-HSCT with 80.6 points and at 18 months post-HSCT with 94.4 points. The difference between baseline and the last time-point was statistically significant (P=0.02). A similar trend was observed in the physical functioning domain [11].
Finally, Kelly et al. compared 13 β-TM patients and a group of 268 patients with hematological acquired diseases and analyzed their HRQoL differences before and after HSCT. The patients were evaluated using CHRIS-General domains before transplant and 45 days as well as 3, 6, and 12 months after transplant. Children with hemoglobinopathies had higher physical and emotional functioning scores prior to HSCT but they experienced a similar pattern of recovery to their baseline functioning after three months post-HSCT when compared to children receiving HSCT for acquired conditions [23].
Overall, the quality of studies collected was good except for one that had low scores (Table S2).
3.2. Quantitative Analysis
Only the comparison between HSCT and transfusion-dependent β-TM patients was considered for quantitative analysis. The analysis was based on 3 HRQoL principal domains: physical, emotional, and social function, and a total of 5 articles were included with 439 patients collected [12, 15-17, 20]. The forest plots with the results are shown in Fig. (2). The random-effect analysis of physical function revealed a significant difference between transplant β-TM and transfusion-dependent β-TM patients, with a medium effect size [d=0.65, 95% CI (0.29-1.02), z = 3.52, p =0.0004]. For emotional function, the effect size was medium again, with significant difference [d=0.59, 95% CI (0.43-0.76), z = 6.99, p <0.00001]. Significant results were found in the social function domain but with a small effect size between the groups [d=0.38, 95% CI (0.02-0.74), z = 2.05, p =0.04]. There was evidence of substantial heterogeneity in the physical and social function domains (I2=75% in both domains) but not in the emotional domain (I2=0%). The risk of bias was evaluated in all of the studies and scores were good except for one [25].
4. DISCUSSION
β-TM can currently benefit from a valid support therapy (transfusion and iron chelation) that, if well conducted, offers a life expectancy similar to that of the healthy population. HSCT is the only treatment capable of assuring definitive healing. Unfortunately, the choice of a transplant procedure in a non-malignant chronic disease is not easy, because of the risk of transplant-related mortality and the clinical complications following HSCT [30]. Ensuring a better HRQoL after HSCT could therefore represent one of the elements that need to be taken into account for the clinical decision-making process. However, few studies have assessed HRQoL in β-TM patients’ literature, and the samples examined frequently differ in age, type of comparison, and questionnaire used. (Fig. 1) describes the article selection process.
HRQoL of transplanted β-TM patients was not different when the comparison was made with other patients transplanted for other conditions, i.e., non-hemoglobinopathies [23]. As expected, in comparison to the general population, lower scores were found in the general health scale [24], but not in the physical health scores [29]. After the transplant procedure, there was a significant increase in HRQoL profile, especially in well-being and physical function [11].
Age at the time of transplantation has a crucial influence on HRQoL. In general, β-TM patients show more anxiety and depression as they age [21]. A comparison of HSCT β-TM children and transfusion-dependent patients revealed lower scores in different domains in the conventionally treated cohort with the worst results found when comparing adults β-TM transfusion-dependent patients [25]. An analysis made in patients aged between 5 and 18 years showed a higher average HRQoL in the transplant group compared with standard support therapy. Sub analysis confirmed differences in the emotional domain in 5 to 7-year-old patients and in the physician and school domains in the 8-12-year-old group [26]. Even distinction between groups of transplanted patients aged more or less than 15 years was evaluated. Worse scores were observed in the patients >15-years for general health and physical component summary [24]. Conversely, other authors comparing patients aged less than 18-years (median age 11.1 years, 5.3-17.9) and patients aged more than 12-years (median age 18.2 years, 12.3-30.4) found significant differences in the second group. In particular, they found differences in overall health and overall physical health between transplant and transfusion groups. Similarly, personal relationship score was higher in transplanted patients. In the group of only pediatric patients, better scores were reported in the running domain and sports exercises but they had lower scores in school absence due to frequent access to the hospital [28] because they needed to attend regular follow-up in the post-transplant period.
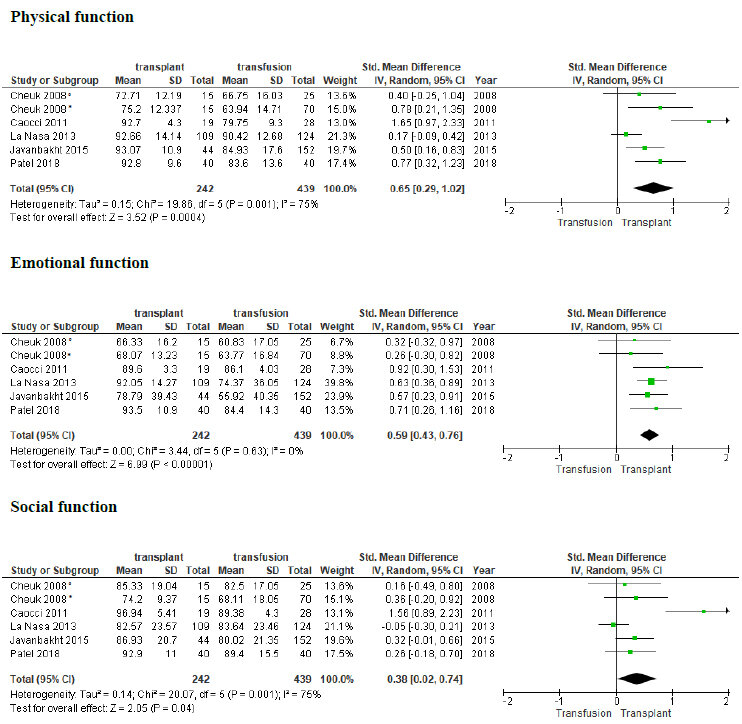 |
Fig. (2). °patients aged < 18 years, * patients aged ≥12 years. |
Besides age, another important factor influencing a good quality of life is GVHD. GVHD decreases HRQoL even after 2 years since transplantation in children and a similar reduction was reported in parents’ score [26]. Differences between the groups with or without GVHD were also present in the physical function, emotional function, pain, and insomnia [22]. Interestingly, HSCT β-TM patients without GVHD in mental health, role emotional functioning, and mental component summary had higher scores compared with the general population [24], suggesting that HSCT is considered as a possible new beginning in these patients.
A more complete vision, however, is given by the analysis between HSCT and transfusion dependent β-TM patients. Overall, analysed HRQoL domains were better in transplanted patients (Fig. 2). In particular, higher scores were found in overall health [26, 28]. Interestingly, all studies recorded higher significant scores in physical domains, ranging between 68.4 and 116.7 [21, 24-29]. The effect size of 5 articles considered in the meta-analysis confirm this trend. Social relationship and psychosocial domains also showed better results [25, 27, 28]. The social function domain showed a significant result but caution in interpretation is necessary because of the small effect size and high heterogeneity. The most interesting results are those related to the emotional function. Indeed, 4 papers reported better significant scores in transplanted patients [10, 13, 15, 16]. Data are corroborated by pooled analysis with medium effect size with significant difference and weak heterogeneity. A recent meta-analysis comparing HRQoL between transfusion dependent β-TM and general population found that patients transfusion dependent β-TM had lower physical and mental health scores [31]. This confirms that the pathology negatively affects different aspects of life. The transplant could fill these gaps and improve the patient’s quality of life.
The current analysis has several limitations. First, only few papers are available for this analysis. The power of results was thus limited. There was heterogeneity in questionnaires utilized. Because some data were missing, meta-regression was not applicable in exploring the related factors such as sex, age, ferritin values. However, the analysis of the available data has emphasized the positive role of transplantation in this category of patients, which has been shown to improve HRQoL.
CONCLUSION
The results of the present study suggested that HSCT in β-TM is associated with positive outcomes in different domains of HRQoL. Novel therapeutic strategies arise in the panorama of β-TM treatments [32, 33]. In this contest, HRQoL appears to be a good instrument for ensuring a better treatment decision. Prospective studies should be conducted to confirm the results obtained.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
This article has been written using PRISMA guide lines. (PRIMSA flow chart and checklist is present on MPS).
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
REFERENCES
| [1] | Caocci G, La Nasa G, d’Aloja E, et al. Ethical issues of unrelated hematopoietic stem cell transplantation in adult thalassemia patients. BMC Med Ethics 2011; 12(1): 4. |
| [2] | Nisbet-Brown E, Olivieri NF, Giardina PJ, et al. Effectiveness and safety of ICL670 in iron-loaded patients with thalassaemia: a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet 2003; 361(9369): 1597-602. |
| [3] | Ladis V, Chouliaras G, Berdoukas V, et al. Survival in a large cohort of Greek patients with transfusion-dependent beta thalassaemia and mortality ratios compared to the general population. Eur J Haematol 2011; 86(4): 332-8. |
| [4] | Porter J. Beyond transfusion therapy: new therapies in thalassemia including drugs, alternate donor transplant, and gene therapy. Hematol Am Soc Hematol Educ Program 2018; 30(1): 361-70. |
| [5] | Shenoy S, Angelucci E, Arnold SD, Baker KS, Bhatia M, Bresters D, et al. Current results and future research priorities in late effects after hematopoietic stem cell transplantation for children with sickle cell disease and thalassemia: a consensus statement from the second pediatric blood and marrow transplant consortium international conference on late effects after pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant 2017; 23(4): 552-61. |
| [6] | Gaziev J, Sodani P, Polchi P, Andreani M, Lucarelli G. Bone marrow transplantation in adults with thalassemia: Treatment and long-term follow-up. Ann N Y Acad Sci 2005; 1054(1): 196-205. |
| [7] | Santarone S, Pepe A, Meloni A, et al. Secondary solid cancer following hematopoietic cell transplantation in patients with thalassemia major. Bone Marrow Transplant 2018; 53(1): 39-43. |
| [8] | La Nasa G, Littera R, Locatelli F, et al. Status of donor-recipient HLA class I ligands and not the KIR genotype is predictive for the outcome of unrelated hematopoietic stem cell transplantation in beta-thalassemia patients. Biol Blood Marrow Transplant 2007; 13(11): 1358-68. |
| [9] | Leplège A, Hunt S. The problem of quality of life in medicine. JAMA 1997; 278(1): 47-50. |
| [10] | Study Quality Assessment Tools | NHLBI, NIH. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools |
| [11] | Caocci G, Efficace F, Ciotti F, et al. Prospective assessment of health-related quality of life in pediatric patients with beta-thalassemia following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011; 17(6): 861-6. |
| [12] | Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. |
| [13] | Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018; 27(6): 1785-805. |
| [14] | Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85(5): 365-76. |
| [15] | McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993; 31(3): 247-63. |
| [16] | Leung KF, Wong WW, Tay MSM, Chu MML, Ng SSW. Development and validation of the interview version of the Hong Kong Chinese WHOQOL-BREF. Qual Life Res 2005; 14(5): 1413-9. |
| [17] | Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med 1998; 28(3): 551-8. |
| [18] | Varni JW, Burwinkle TM, Seid M. The PedsQL 4.0 as a school population health measure: feasibility, reliability, and validity. Qual Life Res 2006; 15(2): 203-15. |
| [19] | Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: Reliability and validity of the pediatric quality of life inventory generic core scales, multidimensional fatigue scale, and cancer module. Cancer 2002; 94(7): 2090-106. |
| [20] | Lucarelli G, Galimberti M, Polchi P, et al. Bone marrow transplantation in patients with thalassemia. N Engl J Med 1990; 322(7): 417-21. |
| [21] | Javanbakht M, Keshtkaran A, Shabaninejad H, Karami H, Zakerinia M, Delavari S. Comparison of blood transfusion plus chelation therapy and bone marrow transplantation in patients with β-thalassemia: Application of SF-36, EQ-5D, and visual analogue scale measures. Int J Health Policy Manag 2015; 4(11): 733-40. |
| [22] | Caocci G, Pisu S, Argiolu F, et al. Decision-making in adult thalassemia patients undergoing unrelated bone marrow transplantation: quality of life, communication and ethical issues. Bone Marrow Transplant 2006; 37(2): 165-9. |
| [23] | Kelly MJ, Pennarola BW, Rodday AM, Parsons SK. Health-related quality of life (HRQL) in children with sickle cell disease and thalassemia following hematopoietic stem cell transplant (HSCT). Pediatr Blood Cancer 2012; 59(4): 725-31. |
| [24] | La Nasa G, Caocci G, Efficace F, et al. Long-term health-related quality of life evaluated more than 20 years after hematopoietic stem cell transplantation for thalassemia. Blood 2013; 122(13): 2262-70. |
| [25] | Patel S, Swaminathan VV, Mythili VS, et al. Quality matters - Hematopoietic stem cell transplantation versus transfusion and chelation in thalassemia major. Indian Pediatr 2018; 55(12): 1056-8. |
| [26] | Uygun V, Tayfun F, Akcan M, et al. Quality of life assessment in hematopoietic stem cell transplantation performed on thalassemia major patients. Pediatr Hematol Oncol 2012; 29(5): 461-71. |
| [27] | Bahar B, Gholibeikian S, Aghdami N, Heshmat R, Safavifar F, Samiee S. Quality of life in beta thalassaemia major at least six months post-allogeneic stem cell transplantation in comparison to the same non-transplanted patients. Bone Marrow Transplant 2005; 35: S268-8. |
| [28] | Cheuk DKL, Mok ASP, Lee ACW, et al. Quality of life in patients with transfusion-dependent thalassemia after hematopoietic SCT. Bone Marrow Transplant 2008; 42(5): 319-27. |
| [29] | Caocci G, Vacca A, Piras E, et al. Return to normal life after hematopoietic stem cell transplantation for thalassemia: a study of patients transplanted from matched sibling donors. Bone Marrow Transplant 2016; 51(12): 1640-1. |
| [30] | Caocci G, Orofino MG, Vacca A, et al. Long-term survival of beta thalassemia major patients treated with hematopoietic stem cell transplantation compared with survival with conventional treatment. Am J Hematol 2017; 92(12): 1303-10. |
| [31] | Arian M, Mirmohammadkhani M, Ghorbani R, Soleimani M. Health-related quality of life (HRQoL) in beta-thalassemia major (β-TM) patients assessed by 36-item short form health survey (SF-36): a meta-analysis. Qual Life Res 2019; 28(2): 321-34. |
| [32] | Cappellini MD, Viprakasit V, Taher AT, et al. A phase 3 trial of luspatercept in patients with transfusion-dependent β-thalassemia. N Engl J Med 2020; 382(13): 1219-31. |
| [33] | Thompson AA, Walters MC, Kwiatkowski J, et al. Gene therapy in patients with transfusion-dependent β-thalassemia. N Engl J Med 2018; 378(16): 1479-93. |








