All published articles of this journal are available on ScienceDirect.
Physical Activity in Depressed Elderly. A Systematic Review
Abstract
Background:
exercise may reduce depressive symptoms both in healthy aged populations and in old patients diagnosed with MDD, but few specific analysis were conducted on the efficacy of exercise as an adjunctive treatment with antidepressants, which may be probably more useful in clinical practice, considered the high prevalence of treatment resistant depression in late life, the low cost and safety of physical activity interventions.
Objective:
to establish the new findings on the effectiveness of exercise on depression in elderlies, with particular focus on the efficacy of the exercise as an adjunctive treatment with antidepressants drug therapy.
Method:
the search of significant articles was carried out in PubMed/Medline with the following key words: “exercise”, “physical activity”, “physical fitness”, “depressive disorder”, “depression”, “depressive symptoms”, “late life”, “old people”, and “elderly”.
Results:
44 papers were retrieved by the search. Among the 10 included randomized controlled trials, treatment allocation was adequately conceived in 4 studies, intention-to-treat analysis was performed in 6 studies, but no study had a double-blinded assessment. We examined and discussed the results of all these trials.
Conclusion:
in the last 20 years, few progresses were done in showing the efficacy of exercise on depression, due in part to the persistent lack of high quality research, in part to clinical issues of management of depression in late life, in part to the difficult to establish the real effectiveness of exercise on depressive symptoms in elderlies. However, there are some promising findings on physical activity combined with antidepressants in treatment resistant late life depression.
BACKGROUND
Major depressive disorder (MDD) represents a common public health issue, it has a lifetime prevalence of 15% to 20% [1, 2] and it has been increased from 15th to 11th rank (37% increase) from 1990 to 2010 among the leading cause of disability in the world [3].
While the prevalence of depressive disorders apparently decreased with age [4], with a prevalence of major depressive disorder in community samples of adults aged 65 and older ranging from 1 to 10% in most large-scale epidemiological studies [5, 6], rates of depression are higher in particular subsets of elderlies, such as medical outpatients (5-10%), medical inpatients (10-12%), and residents of long term care facilities (14 to 42%) [7].
However, it was also observed that the elderly are more involved in the stigma related to depression [8, 9]. It is therefore likely that the elders declare less frequently than non-elderly people depressive symptoms in epidemiological investigations, even when they are suffering. Another aspect concerns the specificity of depressive symptoms in old age. Depression in the elderly, both quantitatively and in terms of the specificity of symptoms, seems to be different from the non-elderly depressive disorders [10]. Indeed, if we investigate the depressive symptoms using diagnostic unstructured tools by means of clinicians, depression rates in the elderly are much higher than by using structured tools [10, 11]. Finally, it has to consider that people who suffer from mood disorders have a lower life expectancy than those who do not suffer from this disorder [12]. So, who has been affected from depression as a young man, is less likely to be older than those who have not suffered. Thus, the notion that the incidence of depression is not frequent in older people is probably an artifact of the epidemiological surveys investigating prevalence, because these investigations have carried out in the general population with rigidly structured interviews and lay interviewers, a method which had been otherwise criticized [13], and are frequently designed to exclude those living in institutions and not at home. This proportion of individuals is high represented in the very old age, which is above all at high risk of depression [14].
It is true, however, that be suffering from depression is a factor that strongly impairs the quality of life and independence of people who are aged [15,16].
The onset and maintenance of depressive disorder in late life seem to follow a vulnerability-stress model, with an interaction between individual vulnerabilities, including genetic factors, age-associated neurobiological and cognitive changes, and a variety of stressful life events that occur more frequently in late life than earlier, such as bereavement, reduction in financial incomes, providing care for an ill relative and occurrence of illness reducing autonomy in daily life [17,18].
Due to the high prevalence of depressive disorder, the efficacy of antidepressants and their low profile of side effects, these drugs have become one of the most common medicaments in the community in western countries, with 6% of utilizers in France [19] and 4.7% in Italy [20]. Randomized clinical trials performed on depressed older adults samples have demonstrated moderate to large effect sizes for selective serotonin re-uptake inhibitors, tricyclic antidepressants and monoamine oxidase inhibitors [21]. Nevertheless, only a small proportion of older adults with depression (around 20%) receive adequate treatment [22, 23]. Lack of treatment among older adults may reflect, in part, the difficulty of detecting depression in older adults, due to age-specific presentation of disease: compared with young adults, older patients tend to present less emotional symptoms of depression, such as sadness, worthlessness/guilt, worry, and fear, and are less accurate at identifying depressive symptoms overall [24]. Moreover, sub-clinical cognitive deficits, as slowness in processing speed and executive dysfunctions, are likely to be showed in elderly patients by objective testing [25], and such deficits may make problematic the psychiatric assessment, reducing rates of diagnosed mental disorder among old people [26]. Anyway, the response rates of antidepressant monotherapy are only from 30% to 45% with single-action or dual-action antidepressant monotherapy [27]. Augmentation therapies or combination with two antidepressants increase the percentage of responders up to 75%, but these strategies increase the risk of side effects and drop-outs [28, 29]. Finally, the presence of serious organic comorbidities may contribute to the depression and complicate the choice of treatment; old patients are likely to use a wide variety of drugs, some of which may worse depression and/or interact with antidepressants, and, metabolizing medications slowly, they are more sensitive to side effects than youngers [30].
Significantly, mortality hazard increases with the severity of depressive symptoms, and antidepressants have not been shown to reduce the mortality rate of elderly patients with persistent symptoms of depression [31, 32].
Because depression in late life, even at sub-threshold levels, is associated with low physical function [33-36], treatment of depression should also provide an opportunity to improve functional limitations [17].
In the last 30 years, a number of studies has suggested that exercise might be effective in preventing or reducing depressive symptoms both in aged healthy populations [37, 38] and as a complementary or alternative treatment for depression [39-41], but this literature has been criticized for methodological weaknesses, such as lack of adequately randomization concealment method and blinding procedure, small samples, and poor quality of data analysis [39].
Nevertheless, considered the interest of researchers on the efficacy and safety of complementary therapies in depressed elderly patients, we thought it might be of interest to review the literature on the topic, in order to establish the role of physical activity in outcomes of depression in late life.
OBJECTIVE
We carried out a systematic review to establish the new findings on the effectiveness of exercise on depression in elderly population, with particular focus on the efficacy of the exercise as an adjunctive treatment with antidepressant drugs therapy.
METHOD
Identification of the Studies
The search of the significant articles was carried out in PubMed/Medline with the following key words: “exercise”, “physical activity”, “physical fitness”, “depressive disorder”, “depression”, “depressive symptoms”, “late life”, “old people”, “elderlies”. Interval was set from January 1990 to December 2012, and the search was further refined on January 2013.
Inclusion Criteria
Studies were included in this review if they were randomized controlled trials, in which exercise was compared to standard treatments (including antidepressant drugs), no treatment or placebo-control, in people aged >60 years old, with depression (diagnosed by any method) as defined by trial authors. We excluded studies different from randomized controlled trials, those that compared different type of exercise without a no-exercising control group, those without an outcome measure of depression, those that measured outcomes immediately before and after a single bout of exercise, samples with mixed diagnosis (i.e. psychiatric and organic comorbidity).
Quality of Studies
We assessed the quality of studies by noting the concealment of allocation, the intention to treat analysis, and the blinding. Trials were distinguished in adequately concealed (if they performed central randomization at a site remote from the study; computerized allocation in which records are in a locked, unreadable file that can be accessed only after entering patient details; the drawing of sealed and opaque sequentially numbered envelopes), or inadequately concealed (open list or tables of random numbers; open computer systems; drawing of non-opaque envelopes). Trials were defined as using intention to treat analysis if all the patients were analyzed in the groups to which they were randomly allocated. For blinding we distinguished between trials in which the main outcome was measured by a blinded assessor and those in which the main outcome was measured either by the participants themselves or by a non-blinded assessor. Moreover, we considered the duration of trials, if the sample had an adequate numerosity, and if it was performed a follow-up assessment. We also considered the quality of assessment (i.e. structured interview, self-report or observer-administered questionnaire), both at the baseline and at the end of trials.
Outcome Measures
We considered as outcome measure the main outcome declared by authors. Because the focus of this paper, we didn’t consider secondary outcomes.
RESULTS
Forty-four papers were retrieved by the search. Thirty-four papers were excluded because these didn’t fulfill our criteria. The abstract of the extracted papers were read and the more pertinent ones (n = 10) were obtained in full version and analyzed in deep. We also examined bibliographies.
Of the 34 papers excluded, 10 were systematic reviews [17, 39-47], most relevant of which we included in session “discussion”. One paper was a commentary [48], 4 did not have an outcome measure of depression [49-52], 2 were on the effect of a single bout of exercise [53, 54], 8 compared different types of exercise but had no non-exercising group [55-62], 2 were conducted on a healthy population [63, 64], 3 had mixed diagnosis samples [65-67], 4 were experimental non-randomized controlled trials [68-71]. Fig. (1) shows the process of inclusion of studies for review.
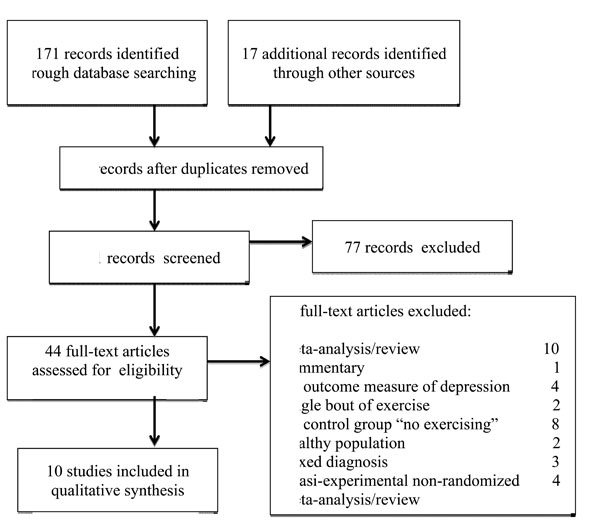
Process of inclusion of studies for review.
Main characteristics of included studies are shown in Table 1.
Characteristics of Included Studies
| Trial | N | Treatment | Control | Duration | Blinding | Assessment | Itt | Results |
|---|---|---|---|---|---|---|---|---|
| Blumenthal et al. 1999 | 156 | Aerobic exercise or Aerobic exercise+ Sertraline | Sertraline | 16 weeks | observer | HAM-D 17 BDI | yes | HAM p=0.39 BDI p=0.40 |
| Kerse et al. 2010 | 193 53.1%* | Home-based exercise [+ antidepressants 28.8%] | Social visits [+ antidepressants 24%] | 12 months | observer | GDS | no | p=0.916 |
| Mather et al. 2002 | 86 | Aerobic supervised exercise + antidepressants | Health talks + antidepressants | 10 weeks + follow-up 34 w | observer | HAM-D17 | yes | p=0.05 [M-W p=0.28] |
| Matthews et al. 2011 | 424 23.8%* | Physical activity intervention | Health talks + stretching | 12 months | observer | CES-D | no | p=0.852 |
| McNeil et al. 1991 | 30 | Supervised exercise | Social contact or Waiting list | 6 weeks | no | BDI | no | p<0.05 [exercise vs waiting list] |
| Sims et al. 2006 | 32 | Strengthening exercise | Brief advice | 10 weeks+ follow up 6 months | observer | GDS | yes | p=0.59 p=0.08 [follow-up] |
| Singh et al. 1997 | 32 | Supervised progressive resistance training | Attention group | 10 weeks | observer | HAM-D BDI |
yes | p=0.008 p=0.002 |
| Singh et al. 2001 | 32 | Supervised weight lift | Health educ. lectures | 20 weeks + follow-up 26 m | observer | BDI | yes | p<0.05 |
| Singh et al. 2005 | 60 | Supervised progressive resistance training high vs low intensity | Usual GP care | 8 w | observer | HAM-D | no | p=0.03 high intensity vs low intensity and usual care |
| Teri et al. 2011 | 273 30%* | Physical activity program [SPA] or SPA+ educational health promotion program | Educational health promotion program or Routine medical care | 3 months + follow up 18 m | observer | GDS | yes | p>0.05 |
* Percentage of depressed patients in the sample.
We found 10 studies that fulfilled our inclusion criteria [72-81].
Of the studies, 6 were carried out in USA [72, 75, 78-81], and one each was in New Zealand [73], UK [74], Canada [76], and Australia [77]. All the included trial but one [74] had intervention groups with supervised exercise programs, compared with health talks, health lectures or brief health advice control groups [74, 77, 79]; Matthews et al. [75] combined health talks with stretching, Teri et al. [81] had a two arms control group, with a program of educational health promotion or routine medical care, McNeil and colleagues’ trial had 2 control groups, with social contact or waiting list [76]; Singh et al. (2001) [79] had an attention control group, while Singh et al. (2005) [80] had a two arms treatment (supervised anaerobic training at high or low intensity) compared to a “usual GP care” control group. Blumenthal et al. [72] performed a trial with a control group on antidepressant therapy with sertraline, Mather et al. [74] had both treatment and control group with antidepressants, Kerse et al. [73] had a fair percentage of participants who undergoing antidepressant therapy both in treatment and in control group.
Quality Assessment
Treatment allocation was adequately conceived in 4 studies [73, 74, 78, 79].
Intention to treat analysis was performed in 5 studies [72, 74, 77-79].
A double-blinded assessment of the main outcome was not performed in any study.
Main outcome was a significant reduction compared to the baseline of GDS score in three studies [73, 77, 81], of BDI score in 2 studies [76, 79], of the HAM-D score in 2 studies [74, 80], of the CES-D score in one study [75]. Two studies [72, 78] assessed participants both with an observer-administered questionnaire (HAM-D) and with BDI.
Exercise Compared to Antidepressants
Blumenthal and colleagues [72] carried out a randomized controlled trial to establish the effectiveness on depression of an aerobic exercise program (3 supervised exercise sessions per week, in a group-setting) compared to sertraline (50-200 mg) or combined treatment (exercise plus sertraline) on a sample of 156 depressed volunteers aged ≥50 years (mean age 57 years). After 16 weeks of treatment, all groups exhibited statistically and clinically significant reductions on HAM-D and BDI scores, without a statistical difference across groups (p= 0.67); adjustment for baseline levels of depression yielded an identical result. Patients receiving medication alone and mildly depressed patients receiving combined treatment exhibited the fastest initial response; however, at the end of treatment, exercise was equally effective in reducing depression.
Exercise Compared to Health Education
Mather and colleagues [74] published the findings of a trial focused on the effect of exercise as an adjunct to antidepressants in reducing depression in a sample of 86 patients aged > 60 years old, poorly responders to antidepressant therapy alone. Participants were randomly assigned to an exercise class group (endurance, muscle strengthening and stretching twice per week, for 10 weeks), or to a control group, with twice-weekly health education talks. Patients were assessed on baseline, 10 weeks and 34 weeks with HAM-D17; because the study focused on a particular population (i.e. a group who had failed to respond to initial treatment), the general convention in trials of antidepressant therapy to use a ≥ 50% reduction in HAM-D17 score as the definition of a response was modified by authors, which assumed that a ≥ 30% reduction in HAM-D17 score associated with participation in exercise could reach clinical interest. At 10 weeks, the exercise group achieved a higher response compared to the control group (p=0.05). Further analysis using the Mann-Whitney test revealed no discernible difference between the two groups in overall effect on the HAM-D17 score (p=0.28).
Matthews et al. [75] carried out a post-hoc analysis of data from the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study, which was a 12 months single-blind randomized controlled trial comparing a moderate intensity physical activity intervention (PA), consisting of a combination of aerobic, strength, flexibility and balance exercises along three phases (individualized, center-based and finally home-based exercise program, with group-based counseling sessions), with the Successful Aging control (SA), a series of sessions on health topics relevant to older adults followed by 5-10 minutes of stretching. Participants were 424 aged volunteers (mean age 76.77 years). Of the sample, 23.8% had depressive symptoms, with 15.8% had high depressive symptom scores (CES-D ≥ 14). The number of participants taking antidepressant medication was significantly higher in those with high depressive (42.1% in PA intervention group, and 55.2% in SA control group) versus low depressive symptoms (18.9% in PA intervention group, and 19.8% in SA control group), but comparable between the two intervention arms. There was no significant improvement in CES-D score over time as a result of participation in either intervention group (p=0.852). No significant changes in CES-D scores were found associated with either intervention when examined in participants with high depressive symptoms (p=0.385) and low depressive symptoms (p=0.670) over the duration of the trial.
Sims et al. [77] performed a trial on 32 depressed volunteers aged ≥ 65 years, randomly assigned at an intervention group (three sessions a week for 10 weeks of moderate intensity strengthening exercises) or at a brief advice control group. Currently receiving antidepressants was an exclusion criterion. Ten weeks follow-up data analyses revealed no significant differences between GDS scores of the intervention and control groups; at six-months follow up there was a trend for the PRT intervention group to have lower GDS scores than the comparison group, but this finding did not reach significance (p = 0.08).
Singh and colleagues (2001) [79] studied 32 community-dwelling patients with major or minor depression or dysthymia (mean age 71.3 years old) in a 20-week, randomized, controlled trial, with follow-up at 26 months. Participants were assigned at the intervention (10 weeks of supervised weight-lifting exercise followed by 10 weeks of unsupervised exercise) or control group attending educational lectures for 10 weeks. The BDI was significantly reduced at both 20 weeks and 26 months of follow-up in exercisers compared with controls (p <0.05 and p=0.001 respectively).
Recently, Teri et al. [81] performed a single-blinded, randomized controlled trial with 2 × 2 factorial design to evaluate the efficacy of a physical activity program (Seattle Protocol for Activity: SPA, consisting in 9 weekly 60 minute sessions followed by 2 bi-weekly 60 minute sessions over 3 months, followed by three monthly and two quarterly booster sessions, for a total of 14 sessions over one year of aerobic/endurance exercises) for low-exercising older adults, compared to educational health promotion program (HP, focused on encouraging participants to maintain a healthy lifestyle, and to engage in regular activities), combination treatment (SPA+HP), and routine medical care control conditions (RMC, including advice and support from their primary physicians and community support services). Participants were 273 older volunteers (mean age 79.2 years); 30% of the sample had mild to moderate symptoms of depression. At the 3-month and 18-month assessments there were no significant differences on the GDS for any treatment condition. No covariate changed the significance of outcome variables in the ITT analyses, nor were there differences in affective outcomes for participants with higher levels of baseline depression (GDS > 8).
Exercise Compared to Health Education
Kerse and colleagues [73] published the findings of a trial focused to establish the effectiveness of a home-based physical activity program (the Depression in Late Life Intervention Trial of Exercise: DeLLITE), in improving function, quality of life, and mood in older people with depressive symptoms. Participants (193 depressed people, aged 75 years and older, mean age 81 years) randomly received either an individualized physical activity program (home-based moderate-intensity balance retraining, progressive resistance lower limb-strengthening exercises, and walking) or social visits (without psychotherapeutic components) over 6 months, with a 12-month follow up. Of the sample, 53.1% had moderate to high levels of depression according to ICD-10, DSM-IV (from CIDI), or GDS-15 criteria, and 26.4% were taking antidepressants, as assessed at baseline. GDS-15 scores improved for all participants over the year of the trial (p<0.001), but there was no significant difference between the 2 groups (p=0.916). There was no differential effect when comparing GDS change in higher depression score participants between the groups over time (p=0.269).
McNeil and colleagues [76] in 1991 carried out a trial on a small sample of 30 elderly volunteers affected by moderate depression, randomly assigned to a 6 weeks intervention of supervised 3-weelkly walking (intervention group), a social contact (home visits by a psychology student twice a week) or a waiting-list control groups. Exercise and social contact both resulted in significant reductions in both the total and the psychological subscale of the Beck Depression Inventory (BDI), without significant difference between these groups; exerciser participants showed a significant difference compared to wait list (p<0.05), and, unlike both the control conditions, showed a decrease also in somatic symptoms subscale of the BDI.
Exercise Compared to Attention Group
Singh et al. (1997) [78] performed a 10 weeks randomized trial on a sample of 32 elderly volunteers (mean age 71.3 years) with major or minor depression or dysthymia, allocated to either a supervised 3 weekly weight-lift intervention group or to an attention control group (seminars on health of elderly people twice a week), assessed by BDI and HAM-D. Anaerobic exercise performed by treatment group significantly reduced all depression measures compared to control (BDI p= 0.002; HAM-D p= 0.008). Interestingly, in a multiple stepwise regression model, intensity of training was a significant independent predictor of decrease in depression scores (r2 = 0.617, p=0.0002).
Exercise Compared to General Practitioner Usual Care
Singh et al. (2005) [80] published the findings of a 8 weeks randomized controlled trial on 60 volunteers aged >60 years old, either allocated in two intervention groups (high versus low-intensity 3 weekly supervised weight training) or standard care by their general practitioner. Authors concluded that high-intensity training group achieved a better response, as assessed by HAM-D score, than low intensity or control group (p=0.03).
DISCUSSION
Due to the variety in samples, characteristics of intervention and control groups, duration of trials, main assessment and follow up, a direct comparison between studies is difficult. Moreover, a lack of adequately concealed randomization, intention to treat analysis and double-blinded design affected the studies. Despite evidence from well-designed studies is limited, a large majority of the selected studies showed significant positive findings in terms of reductions in depressive symptoms obtained by exercisers when compared with controls [72, 74, 76-80]. The few remaining studies showed non-significant trends towards positive outcomes, or a reduction of depression for both intervention and control groups. Thus, we can conclude that physical activity interventions appears to exert beneficial clinical effects on depressive symptoms in elderlies, justifying the interest of researchers in this field, and clinicians’ general conviction.
The latest guidelines from the National Institute for Health and Clinical Excellence [82] included physical activity as a management strategy for depression, recommending structured, supervised exercise programs, three times a week (45 min to 1 h) over 10–14 weeks, at low-intensity, as a Step 2 intervention for mild to moderate depression. Moreover, the guideline for promoting mental health prescribes an accumulation each week of a minimum of 150 minutes of exercise at moderate intensity or a minimum of 75 minutes at vigorous intensity, in bouts of at least 25 minutes over 3 to 5 days per week [83].
Exercise has been supposed acting on depression with a variety of neurobiological effects, such as increase of endorphin and monoamine levels or reduction in the levels of cortisol in the brain [84, 85]. Since it has been hypothesized that depressive disorders might be linked to decreased hippocampal neurogenesis [86, 87], laboratory researches have shown that exercise promotes adult hippocampal neurogenesis [88-90] and triggered dendritic remodeling [91], and such effect exercise-related has been found to be much stronger than that determined by antidepressant drugs [92].
Exercise has been shown to improve subjective quality of life in physical domains in depressed patients [93-95], with higher doses of physical activity associated with larger improvements both in mental and physical domains of QOL. Moreover, exercise may promote other cognitive mechanisms related to subjective wellbeing, as diversion from negative thinking, and a sense of purpose [96, 97].
A series of reviews of literature and meta-analysis, carried out in the recent past, highlighted the interest of researchers in establishing the efficacy of exercise on depressive disorders in the elderlies [17, 40, 42-48, 98], but no specific analysis were conducted on the efficacy of exercise as an adjunctive treatment in old patients who undergoing antidepressant therapy, which may be probably more useful in clinical practice.
A large majority of reviewers found significant methodological limitations in trials, and, in order to recommended exercise as an alternative to empirically validated pharmacological and psychological therapies, claimed for further studies of higher quality [43, 45-47, 99, 100]. Forsman, Nordmyr & Wahlbeck, reviewing the literature on psychosocial interventions for the mental health among older adults, concluded that physical activity had no statistically significant pooled effects on any outcomes with available data when compared with no intervention [101].
The major issue is the relative rarity of randomized clinical trials, which could provide an experimental evidence of the effect of exercise, excluding confounding factors.
Another frequently missed evidence is a dose-response effect, and the minimum effect dose [102]. An expert consensus conference claimed for experimental evidence for a dose–response between physical activity and human health [103, 104]; Dunn, Trivedi, & O’Neal [105] confirmed the lack of studies, but stated that a dose-response relation remains plausible. Ströhle, in a more recent review [106], claimed for further details on the optimal type, intensity, frequency and duration of exercise treatment, highlighting the need of knowledge on how to best deal with depression and anxiety related symptoms, which are likely to hinder patients to be involved in exercise training.
Moreover, many studies on the topic are lacking of a “placebo-control” condition. Klein [107] assumed that researchers who compare non-pharmacologic interventions to antidepressant drugs must include a pill-placebo control group in order to exclude confounding factors; Walsh and Sysko [108] reminded that the proportion of patients responding to placebo in studies on older individuals was slightly higher compared to studies on younger adults, leading to a significantly reduction in effect size. Interestingly, a higher placebo response rate was predicted by low baseline disorder severity [109] and, in clinical practice, patients with mild to moderate depression are likely to have a better placebo response than in clinical trials [110].
On the other side, only few studies focused on the effect of exercise compared to an antidepressant therapy. Thus, in order to show the effect of confounding factors common to exercise and “control” condition, choose an activity such as relaxation, flexibility or stretching exercise as “control” seems to be an acceptable compromise, rather than a “no treatment” group [93, 111]. In fact, the same exercise might to act on depression through elements other than exercise itself, which are likely to be confounding or, at least, mediating factors, such as socialization, learning new skills, shaping the body, weight-loosing, and creativity, all related to psychosocial skills, generally reduced in depressed patients [112-117]. Furthermore, aged people are likely to have low levels of social interactions, which are also related to depression [118, 119], determining time spent in groups or activity programs a treatment per se. Consequently, also social contact seems to be acceptably used as control for the social component of physical activity programs [73].
In a meta-analysis published in 2009, including only randomized controlled trials, Blake and colleagues [42] found important methodological weakness in each of the 11 studies analyzed: allocation was adequately concealed in 5 studies, intention to treat analysis in 5, assessment of outcome was blinded in 7 and the main outcome was measured by self-assessment in 4. Out of 10 included randomized controlled trials, we found important methodological weakness in all. The first problem is the lack of a non-volunteers sample. As observed by Lawlor & Hopker [43], the use of non-clinical volunteers, and the lack of intention to treat analysis suggest that results may overestimate what would be likely in real life.
Among included trials, only one [80] focused on the effect of different intensity exercises, showing high intensity exercise superior compared to control treatment, with no effect for low intensity training. In a study on exercise as adjunct to antidepressants in the treatment of non-remitting depressed patients, Trivedi and colleagues [120] showed that while both high and low exercise doses combined with SSRI treatment produced a decrease of depressive symptoms, there was a trend for higher remission rates in the higher-dose (p<0.06) compared to the lower-dose group. Nevertheless, participants assigned to the low-dose exercise group showed better adherence than those assigned to the high-dose exercise group, showing that low-dose exercise may be both more tolerable and acceptable for depressed patients, and suggesting that in patients in treatment with antidepressants, even low dose of physical activity might be effective in reducing depressive symptoms. A recent study by Callaghan and colleagues [121] seemed to reinforce these findings, affirming the superiority of preferred intensity rather than prescribed intensity exercise in reducing depression.
The effect of exercise on mood as an adjunction to antidepressant therapy was detected in four of the included papers, with different findings: the two stronger trials, carried out by Kerse and colleagues [73] and Matthews et al. [75], which showed no effect, were performed with samples only in part depressed (53.1% and 23.8% respectively) and taking antidepressants, but a separate analysis for depressed subsamples was conducted, which confirmed that there was no significant difference between treatment and control groups (p=0.916 and p=0.385 respectively).
The Blumenthal and colleagues’ trial [72], that showed an identical effect of antidepressants, aerobic exercise and combined treatment, involved a middle aged sample rather than a “older adults” one, as defined by Authors; furthermore, it had a short term duration (16 weeks), without follow-up, and lacked of a “no-treatment” control condition. The trial carried out by Mather et al. [74] appears more interesting, because the mean age of participants (63.7 years in the treatment group, 66.2 years in the control group), and the clinical characteristic of the sample (patients who failed to respond to antidepressants). Two trials carried out on middle-aged populations with treatment-resistant depression showed analogue findings. Pilu et al. [111] performed a randomized trial with naturalistic control on a small sample of 30 depressed women [aged between 40 and 60 years old], undergoing and not responders to antidepressant therapy, randomized either to the treatment (one hour 2 weekly supervised aerobic exercise session plus usual antidepressant) or control group (antidepressant alone) during 8 months, assessed with HAM-D. Only the treatment group showed significant reduction in HAM-D scores (p<0.0001), while controls didn’t improve significantly (p=0.28). Mota-Pereira and colleagues [122] carried out a trial on 33 patients (aged between 26 and 60 years, mean age 45.33 years control group, 48.68 years treatment group) affected by treatment-resistant major depressive disorder. Participants were randomized to the treatment (5 weekly home-based aerobic exercise, consisting in 30-45 min/day walks, as an adjunction at the usual antidepressant treatment) or control group (usual antidepressant treatment alone) for 12 weeks. The treatment group showed lower HAM-D17 rates compared to the control group (p < 0.014). While in the control group none of participants showed response or remission, in the treatment group there were 21% of response and 26% of remission, although these data were not significant.
Thus, while the findings of the latters cannot consent to generalize results, they draw attention on a really important issue, since depressed elderlies in a naturalistic contest often undergoing an antidepressant therapy, and treating resistant depression in older patients represent a challenge. In fact, around two thirds of overall considered antidepressant-treated elderly, and 46% of newer antidepressant-treated patients were considered responders, but older people are particularly vulnerable to side effects: classical TCAs are associated with a higher withdrawal rate due to side effect experience [123], and are more likely to determine acute cognitive impairment related to anticholinergic effects [124]. Moreover, TCAs and MAOIs are associated with severe pharmacodynamic interactions with many medications frequently prescribed to elderly patients [125]. Newer antidepressants, such as escitalopram and duloxetine, are generally well tolerated, but 5-20% and 10-27% of patients, respectively, drop out because of medication-related adverse effects [126]. Rates of treatment resistance in randomized controlled trials in late life depression are as high as 77% using SSRIs and range from 55% to 81% using SNRIs [127-130]. Lithium augmentation in late life antidepressant-resistant patients showed lower rate response and higher side effects than in younger patients [131]. Furthermore, there was limited support for adding an atypical antipsychotic to the antidepressant, and special attention should be addressed to patients with diabetes, dyslipidemia, obesity, Parkinson's disease, QTc prolongation or congestive heart failure, cognitive impairment, constipation, xerophthalmia, and xerostomia [132].
Interestingly, a growing body of evidences links neuropathological correlates of depression, such as hippocampal atrophy, and effect of physical activity on brain plasticity. Smaller hippocampal volumes are associated with major depressive disorder in both young and older populations [133], and also predict poorer long-term outcomes of antidepressant therapies [134]. There is strong evidence that BDNF protein expression plays an important role in the pathophysiology of depression [135], supporting the notion that clinical improvement in depression are associated with neuroplasticity. Both antidepressants [136, 137] and exercise [138, 139] have been shown to block or even to reverse hippocampal atrophy depression-related by increasing BDNF. Vascular disease in the elderly are closely associated with depression [140]. The presence of sub-threshold manic symptoms that is very difficult to identify in the elderly also with the methods of screening [141], increases the risk of vascular dysfunction [142], and thus it is possible that among people with more severe depression they are cases more vulnerable to switch from mania during the antidepressant drug therapy.
Previous laboratory researches suggested that a combined treatment with exercise-plus-antidepressant produced significant increases of BDNF level whereas antidepressant alone failed, and the combination between exercise and reboxetine led to both rapid (detectable at 2 days) and sustainable to 20 days increases in hippocampal BDNF mRNA expression [143]. It is noteworthy that this effect was demonstrated both in young and in aged rats [144]. Very few studies were carried out on this promising field; nevertheless, physical activity has been recommended in combination with other treatments [145], and, in a pilot study, it was proposed as a lower-cost augmentation strategy to improve residual symptoms of depression and prevent relapses [146]. Thus, while further researches are needed to confirm this hypothesis, physical activity may be regarded with interest as an augmentation strategy to ménage treatment resistant depression in late life.
CONCLUSION
Despite the efforts of researchers, in the last 22 years few progresses were done in showing the efficacy of exercise on depression in late life. It was due in part to the persistent lack of high quality research, in part to the difficult to establish the real effectiveness of physical activity, both from a qualitative and a quantitative view, as a management strategy for depressive symptoms, in part to the specific characteristics of depressive disorder in elderlies. While further studies of high methodological quality are required to produce a scientific evidence of exercise as an effective treatment for depression, some promising findings on physical activity combined with antidepressants in depressed patients resistant to antidepressant drugs therapy should be regarded with attention, considering the low cost, the benefits on global health, and the acceptable risk on old patients.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENTS
Declared none.


