All published articles of this journal are available on ScienceDirect.
Prevalence Estimates, Severity, and Risk Factors of Depressive Symptoms among Coronary Artery Disease Patients after Ten Days of Percutaneous Coronary Intervention
Abstract
Background:
Depression and cardiovascular disorders are significant determinants of health that affect the quality of life and life expectations. Despite the high importance of depression screening among Coronary Artery Disease (CAD) patients, the time being to assess and treat it remains controversial.
Objectives:
This study aims to assess the prevalence estimates and severity of depressive symptoms and determine the risk factors associated with developing such symptoms among CAD patients after ten days of Percutaneous Coronary Intervention (PCI).
Methods:
All patients who underwent elective PCI between October 5, 2019, and Mid-March 2020 and diagnosed with CAD were included in this cross-sectional study. CAD was defined as intra-luminal stenosis of ≥ 50% in one or more of the coronary arteries. Depressive symptoms were screened after ten days of the PCI utilizing the patient health questionnaire-9 (PHQ-9) tool. A linear regression model was used to assess the adjusted effects of independent variables on PHQ-9 scores. Electronic medical records, clinical charts, and PCI and echocardiogram reports were reviewed.
Results:
Out of 385 CAD patients, a total of 335 were included in this study, with a response rate of 87%. The participants' mean (±SD) age was 57.5±10.7 years, 75.2% were males, 43% were current smokers, and 73.7% had below bachelor's education. The prevalence estimates of patients with moderate to severe depressive symptoms (PHQ-9 ≥10) was 34%, mild depression 45.1%, and normal 20.9%. Female gender, low educational level and diabetes mellitus were found to be the significant independent predictors of depression among our cohort with (t(333) = 3.68, p<0.001); (t(333) = 5.13, p<0.001); and (t(333) = 2.79, p=0.042), respectively.
Conclusion:
This study suggests a high prevalence of depressive symptoms among CAD patients after ten days of PCI. Approximately one out of three patients with CAD have significant symptoms of depression, which is an alarming finding for clinicians. Moreover, this study demonstrates a lack of sufficient depression recognition and management in similar study settings. Integration of mental health assessment and treatment among patients with CAD as soon as after PCI is recommended for optimal and effective treatment and to obtain the best outcomes.
1. INTRODUCTION
Depression and cardiovascular disorders are two of the most common diseases worldwide and constitute major growing public health problems that affect the quality of life and life expectations [1-3]. Major depressive disorder (MDD) is one of the leading causes of disability worldwide, and its global prevalence ranges from 10.1% to 12.3% [3, 4]. Previous studies shed light on a possible direct association between various cardiac diseases and stress-related disorders such as depression, exhaustion, and anxiety [5, 6]. Nonetheless, depression is highly prevalent among coronary artery disease (CAD) patients; up to 45% of CAD patients could suffer from clinically significant depressive symptoms, and up to 20% diagnosed with MDD [7-9]. Compared to the general population and those without CAD, CAD patients were found to be two- to three-folds more likely to have a diagnosis of depression [10, 11] and similar to the rates of MDD in patients with cancer [12], and diabetes mellitus (DM) [13].
Depression is not only common in CAD patients but also considered an independent risk factor for major adverse cardiovascular events, associated with poor progression of CAD and increased CAD-related mortality [14-17]. Two systemic reviews and meta-analyses showed that depressed post-myocardial infarction patients have a 2- to 2.5-fold increased risk for all-cause mortality [18, 19]. Both pathophysiologic and behavioral mechanisms could explain the connection between depression and CAD, including inflammation, increased platelet activity and aggregation, and autonomic nervous system dysfunction [20]. Besides, depressed patients are less likely to participate in healthy behaviors, including following a healthy diet, maintaining regular exercise, medication adherence, and stress reduction [20].
Even though safe and effective treatments for MDD are available, depression remains underdiagnosed and undertreated [21]. Besides, the link between depression and cardiovascular diseases was known for decades, described by Malzberg in 1937, demonstrating that patients with severe depression had higher mortality than the general population [22]. The rates of depression recognition and treatment remain low in CAD patients; in a previous study conducted on hospitalized cardiac patients, only 11% of patients with depression received adequate antidepressant therapy [23]. Besides the importance of depression screening among such patients, the controversy may be related to the time of assessment and when should psychopharmacotherapy be given.
This study aims to estimate the prevalence rates of depressive symptoms, evaluate depression severity among CAD patients after ten days of diagnosis, and determine the predictors of developing depressive symptoms among these patients. This study's novelty lies in its time frame as depression screening was performed after a little time of CAD diagnosis.
2. MATERIALS AND METHODS
2.1. Study Design, Patients Participation, and Recruitment
This is a single-centered, cross-sectional study conducted between October 5, 2019, and Mid-March 2020 in the cardiac catheterization center at King Abdullah University Hospital (KAUH), north of Jordan. The study included all patients who were diagnosed with stable CAD with neither previous nor family history of depression, and admitted for elective percutaneous coronary intervention (PCI). Out of 462 patients admitted for elective PCI during the period as mentioned earlier, 385 had a confirmed CAD diagnosis via angiography. CAD diagnosis was defined as intra-luminal stenosis of ≥50% in the left main coronary artery or ≥70% in one or several major epicardial arteries or significant branches (>2 mm in diameter). This definition is consistent with the American College of Cardiology Foundation, American Heart Association, and European Society of Cardiology definitions for CAD [24-26]. Exclusion criteria included patients who: 1) were under the age of 18 years, 2) were already on antidepressant drugs, 3) had a previous or family history of depression before PCI, 4) had depression secondary to substance abuse or any organic disorder as thyroid disorders or cancer, or 5) presented with the acute coronary syndrome. Also, subjects with congenital heart disease or who were unwilling to consent were excluded. Patients who showcased the following health complications after PCI: intubated, in shock, transferred to the intensive care unit, acute pulmonary edema, fatigued, or in active pain were also excluded.
Electronic medical records, clinical charts, PCI reports, and nursing records of all included patients were reviewed, and detailed socio-demographic information, comorbidities, previous history of CAD, and PCI findings were recorded for participants who consented to the study. A review of the Echocardiogram obtained the ejection fraction.
Participants' risk and severity of depressive symptoms were assessed using the Arabic version of the 9-item Patient Health Questionnaire (PHQ-9) after ten days of the PCI [28]. The validity, reliability, and accuracy of this tool were assessed elsewhere [27-30]. PHQ-9 consists of nine items following the MDD diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV). Participants’ responses were coded using a 4-point Likers scale: 3 = nearly every day; 2 = more than half of the days; 1 = several days; 0 = not at all. Item points (0-3) were added to the total score depending on the patient's choice for each item. A final score ranging between 0 and 27 was calculated for each patient. Higher scores indicated increased depression severity. PHQ-9 is a dual-purpose instrument that can grade the severity of depressive symptoms and establish MDD diagnosis risk [29, 30]. The total score of the PHQ-9 questionnaire for each participant was interpreted as follows: no (0-4), mild (5-9), moderate (10-14), moderately severe (15–19), and severe (20-27) risk of depression [29, 30]. PHQ-9 score ≥10 had a sensitivity of 88% and 85-88% specificity for the MDD [29, 30].
2.2. Ethical Standards
This study was approved by the Institutional Review Board (IRB) of Jordan University of Science and Technology and King Abdullah University Hospital (IRB reference number is 36/132/2020). Signed informed consent was collected from each participant before enrollment. The survey was anonymous, and the recruited information was kept confidential. This study was conducted following the 1964 Helsinki declaration, as revised in 2009 and its amendments or comparable ethical standards [31]. The study protocol was registered at the Registry website with a unique identification number of 6106 [32].
2.3. Data Statistical Analysis
Data were analyzed and proceeded using the IBM Statistical Package for the Social Sciences (SPSS) software for Windows, version 25.0 (Armonk, NY: IBM Corp). This was important in establishing the central tendencies, dispersion, variance, and skewness of the data. Categorical data variables were described using frequency and percentage, while continuous data variables were presented using mean and standard deviation (SD). An independent sample t-test, or one-way ANOVA was conducted to examine the variances in depression scores based on the demographic and clinical-comorbid variables. Similarly, multiple-linear regression analysis was employed to assess the adjusted effects of independent variables on PHQ-9 scores. A p-value of less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Demographical Details
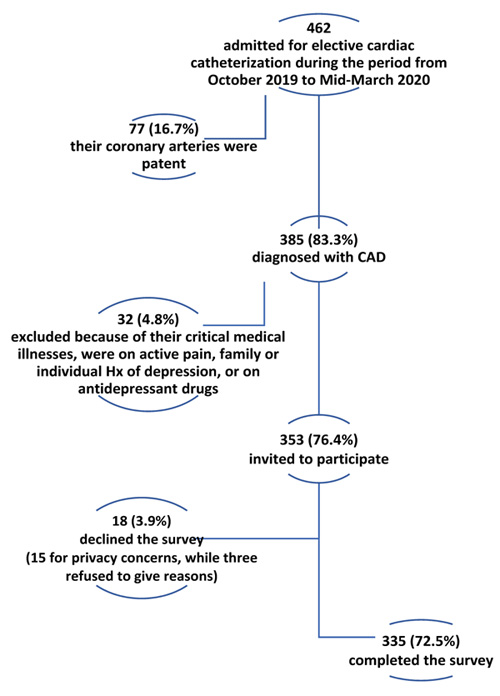
Out of 385 CAD diagnosed patients, a total of 335 patients were included in this study, with a response rate of 87% (Fig. 1). The participants' average (±SD) age was 57.5 (±10.7) years, ranging between 27 and 87. More than three-quarters of participants (75.2%) were males, 65.4% were unemployed, 73.7% reported having below bachelor's education, and 43% were current smokers. The mean (±SD) body mass index (BMI) was 28.1 (± 3.0) kg/m2, ranging between 20 and 36 kg/m2. The mean ejection fraction was 51.72 (SD=9.69) percent, ranging between 24% and 69%. A high skewness level was found in the ejection fraction variable (-1.18), so it was categorized into ≥45% (79.1%) and <45 (20.9%). The vast majority of participants had comorbidities such as hyperlipidemia, hypertension, and previous history of CAD. More than half of the patients (57.6%) had DM. Detailed demographic information and clinical-comorbid features are summarized in Table 1.
| Factors | Categories | Frequency | Percentage (%) | M (±SD) | Range |
|---|---|---|---|---|---|
| Age | - | - | - | 57.46 (±10.69) | 27-87 years |
| Gender | Male | 252 | 75.2 | - | - |
| Female | 83 | 24.8 | - | - | |
| Education Level | Below Bachelor | 247 | 73.7 | - | - |
| Bachelor’s degree or higher | 88 | 26.3 | - | - | |
| Employment status | Employed | 116 | 34.6 | - | - |
| Unemployed | 219 | 65.4 | - | - | |
| Smoking | Smoker | 144 | 43.0 | - | - |
| Non-smoker | 191 | 57.0 | - | - | |
| Hypertension | Yes | 271 | 80.9 | - | - |
| No | 64 | 19.1 | - | - | |
| Hyperlipidemia | Yes | 295 | 88.1 | - | - |
| No | 40 | 11.9 | - | - | |
| Diabetes mellitus | No | 142 | 42.4 | - | - |
| Yes | 193 | 57.6 | - | - | |
| Previous history of CAD | Yes | 257 | 76.7 | - | - |
| No | 78 | 23.3 | - | - | |
| Ejection Fraction | ≥45% | 265 | 79.1 | 51.72 (±9.69) | 24-69% |
| <45% | 70 | 20.9 | - | - | |
| BMI (Kg/m2) | - | - | 28.10 (±3.00) | 20-36 |
3.2. The Prevalence Rates of Depressive Symptoms
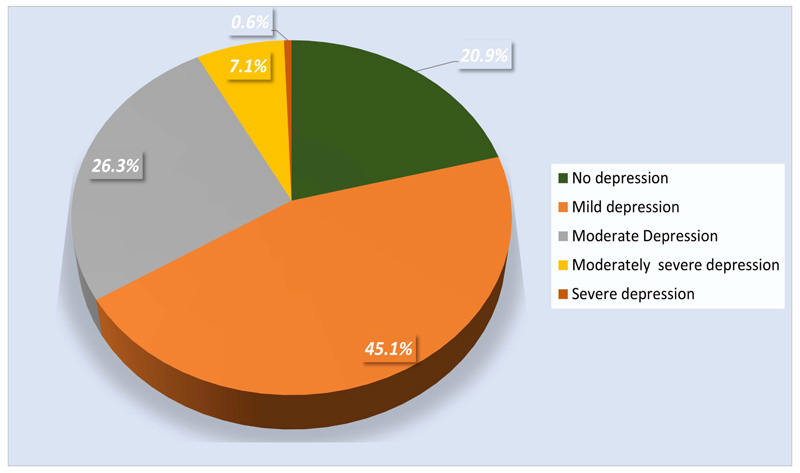
As shown in Fig. (2), Approximately one-fifth of the sample (n=70) reported no depressive symptoms, while 45.1% (n=151) showed mild depressive symptoms. About 26.3% (n=88) had moderate depressive symptoms and 7.2% (n=24) displayed moderately severe depressive symptoms. Additionally, two participants (0.6%) had severe depression risk. Overall, the PHQ-9 mean score was 8.0 (SD=4.1), ranging between 0 and 21. The prevalence estimate of MDD, PHQ-9 score ≥ 10, was 34% (144 out of 335).
| Variable | Categories | PHQ-9 score Mean (±SD) |
t (333) | p-value | 95% CI |
|---|---|---|---|---|---|
| Gender | Male | 7.50 (±3.94) | -4.00 | <0.001 | 1.03 - 3.04 |
| Female | 9.54 (±4.28) | - | - | - | |
| Employment status | Employed | 7.59 (±4.01) | -1.33 | 0.18 | -0.30 - 1.55 |
| Unemployed | 8.22 (±4.16) | - | - | - | |
| Educational level | Below Bachelor | 8.74 (±4.07) | 5.72 | <0.001 | 1.83 - 3.75 |
| Bachelor or higher | 5.94 (±3.50) | - | - | - | |
| Smoking | Smoker | 8.03 (±4.19) | 0.91 | -0.94 - 0.84 | |
| Non-smoker | 7.98 (±4.07) | -0.11 | - | ||
| Hypertension | Yes | 8.26 (±3.98) | 0.01 | -2.49 - -0.26 | |
| No | 6.89 (±4.51) | -2.42 | - | ||
| Hyperlipidemia | Yes | 8.13 (±4.08) | 0.10 | -2.47 – 0.24 | |
| No | 7.02 (±4.25) | -1.60 | - | ||
| Diabetes mellitus | Yes | 8.51 (±4.18) | 0.008 | -2.08 – -0.30 | |
| No | 7.31 (±3.94) | -2.65 | |||
| Previous history of CAD | Yes | 8.08 (±4.07) | 0.52 | -1.39 – 0.70 | |
| No | 7.74 (±4.28) | -0.64 | - | ||
| Ejection Fraction | ≥45% | 7.91 (±4.24) | -0.80 | 0.42 | -0.64 – 1.53 |
| <45% | 8.35 (±3.62) | - | - | - |
3.3. Differences in Depression Score Based on Demographic and Clinical-comorbid Variables
An independent t-test was used to examine the differences in mean PHQ-9 scores by demographical and clinical-comorbid variables (Table 2). Significant differences in PHQ-9 mean scores were detected by gender, educational level, hypertension, and DM. Female participants had significantly (t (333) = 4.00, p<0.001) higher mean PHQ-9 score (9.54 ± 4.28) compared to their male counterparts (7.50 ± 3.94). Participants with below bachelor’s educational level had significantly (t (333) = 5.72, p<0.001) higher mean depression score (8.74 ± 4.07) compared to those with bachelor’s education or higher (5.94 ± 3.50). Participants with HTN had a significantly higher mean PHQ-9 score (8.26 ± 3.98) compared to their counterparts without such comorbidity (6.89 ± 4.51) (t (333) = 2.42, p=0.01). DM participants had significantly (t (333) = 2.65, p=0.008) higher mean PHQ-9 score (8.51 ± 34.18) compared to non-diabetic participants (7.31 ± 3.98).
No significant differences in mean PHQ-9 scores were detected by occupational status (t (333) =1.33, p=0.18); smoking status (t (333) =-0.11, p=0.91); hyperlipidemia (t (333) =0.86, p=0.10); previous history of CAD (t (333) =-0.64, p=0.52); and ejection fraction (t (333) =0.80, p=0.42).
3.4. Predictors of Depressive Symptoms among CAD Patients
A multiple-linear regression model was used to determine the adjusted effects of independent variables, including demographical details and the clinical-comorbid variables, on PHQ-9 scores to determine the depressive symptoms predictors. The entire model was established to be significant and explained 13% of the variance in PHQ-9 scores (F (11, 333) = 5.33, p<0.001). The model is summarized in Table 3.
Female gender showed (t (333) = 3.68, p<0.001), low educational level had (t (333) = 5.13, p<0.001), and diabetic patients (t (333) = 2.79, p=0.042) were significant predictors of PHQ-9 scores after controlling for the other variables in the model. Age, occupation, smoking status, hypertension, hyperlipidemia, previous history of CAD, BMI, and ejection fraction were not significant predictors of PHQ-9 scores (p>0.05 for each variable).
| Variables | Unstandardized β | t (333) | p-value | 95% CI. | Part Square |
|---|---|---|---|---|---|
| Age | -0.03 | -1.40 | 0.16 | -0.08 – 0.01 | 0.49 |
| Gender (male) | -2.06 | -3.68 | <0.001 | -3.16 – -0.96 | 3.57 |
| Occupation | -0.15 | -0.27 | 0.78 | -0.26 – 0.96 | 0.01 |
| Education (Bachelor or higher) | -2.52 | -5.13 | <0.001 | -3.48 – -1.55 | 6.76 |
| Smoking | 0.84 | 1.75 | 0.08 | -0.10 – 1.79 | 0.81 |
| Hypertension | 0.62 | 1.02 | 0.30 | -0.57 – 1.81 | 0.25 |
| Hyperlipidemia | -0.46 | -0.62 | 0.53 | -1.91 – 0.99 | 0.09 |
| Diabetes mellitus | 1.85 | 2.79 | 0.04 | 0.25 – 1.80 | 1.81 |
| Previous history of CAD | 0.18 | 0.33 | 0.73 | -0.90 – 1.28 | 0.02 |
| BMI | 0.04 | 0.59 | 0.55 | -0.10 – 0.18 | 0.09 |
| Ejection Fraction | -0.46 | -0.86 | 0.39 | -1.51 – 0.59 | 0.23 |
| First Author, Year | Location of Study | Setting | Sample Size (CAD Patients) |
Age, Years (mean ± SD) or (range) |
Females, n (%) |
Depression Assessment |
Symptoms of Depression, n (%)* |
|---|---|---|---|---|---|---|---|
| Present study | Jordan | Outpatients | 335 | 57.46 ± 10.69 | 83 (24.8%) | PHQ-9 | 144 (34%) |
| Al-Zaru et al., 2020 [30] | Jordan | Outpatients | 174 | 58.6 ± 10.2 | 84 (48.3%) | CDS | 93 (53.4%) |
| Alkafaji et al., 2020 [31] | Iraq | Inpatients | 120 | 20 - 80 | 44 (36.7%) | BDI | 58 (48.3%) |
| Shiotani et al., 2002 [32] | Asia | Inpatients | 1,042 | 63 ± 11 | (19.4 %) | SDS | 438 (42%) |
| Myers et al., 2012 [33] | Israel | Inpatients | 632 | 52 | - | BDI | 176 (27.8%) |
| Seecheran et al., 2019 [34] | Trinidad and Tobago | Outpatients | 1203 | 62.5 ± 13.1 | 632 (52.5%) | PHQ-9 | 301 (25%) |
| Sharma Dhital et al., 2018 [35] | Nepal | Outpatients | 168 | 53.01 ± 13.91 | 66 (39.3%) | HADS | 40 (23.8%) |
| Davidson et al., 2010 [36] | USA | Inpatients | 453 | 25 - 93 | 190 (42%) | Structured Psychiatric Interview | 109 (24.0%) |
| Ziegelstein et al., 2000 [37] | USA | Inpatients | 204 | 60 | 88 (43.1%) | BDI | 35 (17.2%) |
| Lauzon et al., 2003 [38] | Canada | Inpatients | 550 | 60 | 116 (21%) | BDI | 191 (35%) |
| Kuhlmann et al., 2019 [39] | German | Inpatients | 1,024 | 62.7 ± 10.2 | 197 (19.2%) | PHQ-9 | 463 (45.2%) |
4. DISCUSSION
Medical literature focused on the cardiac treatment of CAD patients and the technic and complications associated with catheterization procedures with less attention to the mental health problems among this population. The current study demonstrated that symptoms of depression were common among CAD patients after ten days of PCI. Also, the prevalence estimate of MDD was high, 34% or about four in every ten patients, as indicated by the PHQ-9 cut-off point of ≥10. Female gender, low educational level, and DM were independent predictors for high PHQ-9 scores, indicating a higher risk of depression. These results suggest that potential depression comorbidity may be under-estimated and underdiagnosed among our CAD patients. These findings are alarming, considering the lack of sufficient depression screening, recognition, and management in similar study settings. Integrating mental health assessment and treatment among patients with CAD, especially in a short time after PCI, is crucial for optimum health. The findings call for harmonization of care between healthcare providers and making changes in the instituting policies to enhance referral and management of potential depression among CAD patients.
Previous studies revealed a relatively high heterogeneity in the prevalence estimates of depressive symptoms among CAD patients, as shown in Table 4. These prevalence estimates’ discrepancy could be attributed to the different methods and designs used in the included studies, such as the different tools for assessing depression. However, these studies, as well as our study, reported a high prevalence of depressive symptoms among CAD patients that ranged between 17% and 53% [33-43].
The prevalence of depressive symptoms among CAD patients in the current study was 34%, which was lower than the previously reported estimates among Jordanian patients with CAD, as they estimated a prevalence rate of 53.4% using a cut-off point of 90 in the Cardiac Depression Scale (CDS) screening tool [33]. This difference in prevalence estimates could be attributed to the different cohorts investigated, methods, tools, and time-frame as the latter study included a smaller sample size with more females than our study. Besides, Al-Zaru et al. included a significant number of patients with cancer and thyroid disorders (31%), which could overestimate the prevalence of depressive symptoms [33]. However, patients with these conditions were not included in our study. Also, the cut-off point of 90 in the CDS has higher sensitivity for depression as it included mild, moderate, and severe depressive patients while our study, using PHQ-9 cut-off point of ≥ 10 for estimating the prevalence estimate of MDD, excluded 45.1% mild depressive patients. Still, the superiority of the other self-report questionnaires over PHQ-9 in the screening of depression remains controversial [44, 45]. While our study was conducted after ten days of PCI, Al-Zaru et al. included patients with a previous CAD history regardless of the time lag between survey and catheterization procedure [33]. These variations may contribute to discrepancies between prevalence estimates reported in the literature and the current study.
On the other hand, our estimated prevalence of depressive symptoms among CAD patients was comparable to the findings of Lauzon et al., where they reported a prevalence of 35% among Canadian CAD patients [41]. Moreover, two studies estimated the prevalence of depression among CAD patients before and after the PCI; Wang et al. [46] used a combination of Hospital Anxiety and Depression Scale and the Mini-International Neuropsychiatric Interview to identify patients with depression, while Li et al. [47] determined depression prevalence based on Zung Self-Rating Depression Scale. These two studies reported depression in nearly 40% of the postoperative patients, increasing more than ten percentage points than before the operation [46, 47].
Several previous works showed that depression is associated with a higher risk of developing cardiovascular diseases [5, 6, 48-50]. A recent study conducted by Daskalopoulou et al. examined depression as a possible risk factor for 12 cardiovascular diseases in approximately 2 million men and women; it was shown that depression was prospectively associated with cardiac, cerebrovascular, and peripheral diseases, with no evidence of disease specificity [6]. Smeijers et al. investigated levels of psychological distress (depressive symptoms, perceived stress, general anxiety), illness-related anxiety, and personality factors among patients with takotsubo cardiomyopathy [51]. Takotsubo cardiomyopathy is an acute and transient condition characterized by left ventricular wall motion abnormality combined with symptoms and signs mimicking acute MI, predominantly in postmenopausal women [52-54]. The authors studied 18 patients with takotsubo cardiomyopathy and two comparison groups: 19 healthy controls and 19 patients with heart failure [51]. It was shown that takotsubo cardiomyopathy was associated with higher levels of depressive symptoms, more illness-related anxiety, and less openness than healthy controls, and No differences between Takotsubo cardiomyopathy and heart failure patients regarding the psychological measures [51]. Other studies concordant with Smeijers et al. findings and reported a significant association between Takotsubo cardiomyopathy and psychiatric disorders such as depression [55-59].
Regarding CAD specifically, two independent reviews found that the relative risk for the onset of CAD was 1.64 for depressed compared to non-depressed individuals followed for an average of 13 years [16, 60]. Studies that have followed large population samples for from 4 to 10 years have found that both major depression [61] and depressive symptoms [62] predict incident CAD in individuals initially free of heart conditions, controlling for common risk factors. Even increasing recognition of this relationship led both American and European treatment guidelines to recommend routine screening for depression and anxiety in CAD patients [63]; this relationship remains underestimated in developing countries.
This study revealed a significantly higher risk of depression among females than males, which is concordant with previous studies' findings [33, 37, 38, 64, 65]. Some studies reported a 1.7-fold higher incidence and prevalence in women [66, 67]. This gender disparity could be attributed to the hormonal (biological) effects, amongst other issues, such as cultural differences, education, and diet [66]. The traditional role of Jordanian women as responsible for their families could be a source of stress. Besides, the housework responsibility of women could increase the risk of depression [38]. Moreover, in Jordanian culture, women's actions are more likely to be criticized than men's. Gender role (masculinity) may also predispose women to be more susceptible to depression compared to males.
Our study did not find significant differences in PHQ-9 scores by age, concordant with a previous study from Jordan [33] and other studies [38, 64]. On the other hand, this finding contradicts Konrad et al.'s study, conducted in Germany, where it was reported that older patients with CAD faced a higher risk for depression than their younger peers [68]. Our finding could be explained by the more religious participation of older people than younger people in Muslim countries such as Jordan, which may help patients mitigate their current conditions.
Besides being female, the study's findings showed that low educational level was a risk factor for developing depression among CAD patients. Likewise, Dhital et al. found that levels of depression were more prevalent among illiterate respondents and those with low educational levels [38]. Also, Al-Abbudi et al., a study conducted in a neighboring country, found that 85% of depressed ischemic heart disease patients were of low educational level or illiterate [65]. In contrast to this finding, a study conducted by the National Heart Association of Malaysia revealed that education level is not associated with depression [69]. This discrepancy in findings might be due to the inclusion of different samples and the study settings.
Regarding comorbidities associated with higher depression scores, our study found that hypertension and DM were associated with higher PHQ-9 scores, based on univariate analysis, which is concordant with Bahall et al. findings [64]. However, after adjusting for potential confounding factors, including demographic characteristics and the clinical-comorbid variables, hypertension did not predict PHQ-9, while DM was the only comorbidity as a significant predictor for higher PHQ-9 scores and subsequent development depression.
Increased depression risk among DM patients was suggested by our findings, which is concordant with a previous study conducted in Jordan, where it was reported that more than one-third of diabetic patients had depression, according to PHQ-9 [13]. A case-control study conducted by Anderson et al. reported that the odds of depression in diabetic patients were twice as high as those in the non-diabetic comparison group (OR = 2.0, 95% CI 1.8–2.2) [70]. Also, Al-Ghamdi et al. concluded that depression is more common among diabetic patients (34%) compared to non-diabetics (13%) (p<0.001) [71]. This finding might be due to more somatic symptoms related to DM, which interrupt the CAD patients' daily activities and caused them to feel more depressed. Besides, the somatic symptoms and behaviors related to diabetes, such as tiredness, sleeping, and poor appetite or overeating, are highlighted in the PHQ-9 but not in others [72].
There are several limitations to this study. First, this study's cross-sectional design addresses only one point in time that lacks temporality and comparison of depression prevalence estimates before and after CAD diagnosis. It also lacks a comparison, control, group. Therefore, we could not provide evidence for a causal relationship between depression and CAD. A prospective cohort will be a better fit to assess this relationship. Second, the number of enrolled patients was relatively small, although the response rate was high; a total of 18 CAD patients declined to participate. This decline could be due to the cultural perspective regarding depression and the social stigma attached to mental health problems, especially in developing countries. Thus, a response bias may have occurred if the non-responders refused the participation as they felt uninterested or depressed. However, at the beginning of this study, we intended to include a larger sample size of CAD patients and make the study design a case-control one, but the COVID-19 crisis that affected Jordan in mid-March restricted such plans [73-77]. Third, PHQ-9 is a screening tool that needs a psychiatrist to conduct a structured clinical interview to confirm depression diagnosis. This could have overestimated the prevalence estimated of depression among our patients with CAD [78, 13]. Fourth, the patients’ truthfulness for their responses to the questions asked is controversial as some responses may not have told the truth on purpose to prevent doctors’ concern for mental health rather than cardiovascular health or that negative patients' stigma to state how they really feel. However, we assured participants of the results' confidentiality to avoid such effects of underestimating the reported depression estimates.
Regardless of the limitations, this study provided baseline estimates for potentially depressive symptoms among CAD patients within developing country settings and established potential predictors for the risk of depression. Future investigations of this research problem should focus on utilizing a follow-up approach and consider psycho-behavioral or pharmacological interventions to estimate the effects of treating depression on cardiac health among CAD patients, assess the benefits of supportive psychotherapy until cardiac condition stable, and better investigate the bidirectional relationship between depression and cardiac health. Larger-scale studies with proper depression assessment are needed to validate our findings and help implement change to no longer understate depression associated with cardiovascular diseases. Investigation of the efficacy of depression treatment in lowering the morbidity and mortality rates secondary to cardiac disease is also a research need.
CONCLUSION
This study suggests a relatively high prevalence estimate of depressive symptoms among CAD patients after ten days of PCI. Approximately one in three patients with CAD have symptoms of major depressive disorder. Female gender, low educational level, and diabetes mellitus were independent predictors for high depression risk among CAD patients. These findings are alarming, considering the lack of sufficient depression screening, recognition, and management in similar study settings. Integration of mental health assessment and treatment among patients with CAD as soon as after PCI is crucial for optimum health. Advanced cohort studies related to depression and the necessity of psychopharmacotherapy in CAD patients are suggested.
LIST OF ABBREVIATIONS
| CAD | = Coronary Artery Disease |
| PCI | = Percutaneous Coronary Intervention |
| PHQ-9 | = Patient Health Questionnaire-9 |
| SD | = Standard Deviation |
| MDD | = Major Depressive Disorder |
| DM | = Diabetes Mellitus |
| KAUH | = King Abdullah University Hospital |
| IRB | = Institutional Review Board |
| SPSS | = Statistical Package for the Social Sciences |
| BMI | = Body Mass Index |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All procedures performed in this study were reviewed and ethically approved by the Institutional Review Board (IRB) and the research and ethics committee at King Abdullah University Hospital and Jordan University of Science and Technology (IRB reference number: 36/132/2020).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1964, as revised in 2009 and its amendments.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all participants included in this study.
AVAILABILITY OF DATA AND MATERIALS
The datasets generated and analyzed during the current study are available with the corresponding author [A.A] and can be provided on a reasonable request according to IRB approval restrictions.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


