All published articles of this journal are available on ScienceDirect.
Assessment of Extrapyramidal Symptoms Associated with Psychotropics Pharmacological Treatments, and Associated Risk Factors
Abstract
Background:
Extrapyramidal Symptoms (EPS) are unwanted symptoms commonly originating from the use of certain medications. The symptoms can range from minimal discomfort to permanent involuntary muscular movements. The aims of the study were to examine the incidence of drug-induced extrapyramidal symptoms (di-EPS), associated risk factors, and clinical characteristics.
Methods:
This is a retrospective, observational study of di-EPS conducted in outpatient clinics of Jordan using the longitudinal health database (Hakeem®) for data collection. Patients who received drugs with the risk of EPS during the period 2010-2020 were included and followed. Patients with any of the known underlying conditions that may cause EPS or were currently taking drugs that may mask the symptoms were excluded. Gender and age-matched control subjects were included in the study. The Statistical Package for Social Science (SPSS®) version 26 was used for data analysis.
Results:
The final dataset included 34898 exposed patients and 69796 matched controls. The incidence of di-EPS ranged from 9.8% [Amitriptyline 25mg] to 28.9% (Imipramine 25mg). Baseline factors associated with a significantly higher risk of developing di-EPS were age {HR: 1.1 [95%CI: 0.8-1.2, p=0.003], smoking {HR: 1.7 (95%CI: 1.3-2.2), p=0.02}, tremor history {HR: 7.4 (95%CI: 5.9-8.3), p=.002} and history of taking antipsychotics {HR: 3.9, (95% CI: 2.5-4.6), p=0.001}. Patients taking paroxetine {HR: 8.6 [95%CI: 7.4-9.8], p=.0002},imipramine {HR: 8.3, [7.1-10.5], p=0.01}, or fluoxetine {HR: 8.2 (95%CI: 6.8-9.3), p=.006} had a significantly higher risk of developing di-EPS compared to patients taking citalopram. Myoclonus, blepharospasm, symptoms of the basal ganglia dysfunction, and organic writers' cramp were reported among participants.
Conclusion:
Patients treated with paroxetine, imipramine, fluoxetine, or clomipramine had a higher risk of developing di-EPS than patients treated with citalopram. The difference in gender was not significantly related to di-EPS development. Whereas age, smoking, and history of taking antipsychotics were significantly associated with di-EPS development.
Key findings:
• High incidence of drug-induced extrapyramidal symptoms (di-EPS) was reported
• Age, smoking, tremor history, and history of taking antipsychotics were risk factors of drug-induced extrapyramidal symptoms.
• Patients taking paroxetine, imipramine or fluoxetine had a significantly higher risk of developing di-EPS compared to patients taking citalopram
1. INTRODUCTION
Extrapyramidal Symptoms (EPS) are undesired adverse reactions frequently developing from the use of Antipsychotic Medications (APMs) and these reactions were firstly described in 1952 [1]. EPS include different movement disorders that can be categorised into acute and tardive syndromes. Acute reactions are those that originate within hours or days of starting APMs and include parkinsonism, akathisia, and dystonia [2, 3]. Tardive dyskinesia and tardive dystonia are chronic manifestations that develop following prolonged administration of APMs [4]. These symptoms are debilitating, interfering with motor tasks, social communication, and activities of daily living. Furthermore, they are associated with poor quality of life and therapy discontinuation, and thus disease relapse and re-hospitalization may occur, particularly in mentally impaired patients [5]. The most common medications associated with EPS are dopamine-receptor blocking agents, especially the first-generation antipsychotics haloperidol and phenothiazine neuroleptics, and to a lesser extent, EPS are associated with atypical antipsychotics. The risk of EPS increases with dose escalation and also other agents may induce EPS, including antiemetics [6], lithium [7], serotonin reuptake inhibitors [SSRIs] [8], tricyclic antidepressants (TCAs) [8], calcium channel blocker [9], and stimulants [10].
In Jordan and the Middle East in general, medication-related problems are common [11-19]. The National Centre for Mental Health was established in 1987, with 350 beds, and there are many outpatient clinics that provide psychiatric services across the country [20]. Given the serious complications of drug-induced EPS and the scarcity of data available about the incidence of drug-induced EPS and associated risk factors in Jordan, we believe our research will provide a better understanding of psychiatry related problems and reliable characterisation of EPS incidence and associated known risk factors in outpatient clinics.
2. METHODS
2.1. Study Design and Data Source
This is a retrospective, 10-year, non-interventional, longitudinal, observational study of drug-induced extrapyramidal symptoms [di-EPS] conducted in outpatient clinics in Jordan. Data were collected using “Hakeem©” database, which is the electronic prescribing system launched by Electronic Health Solutions (EHS) in 2009, and since then, it was used by the ministry of health in Jordan. This database covers clinical information of the Jordanian community in all government health care facilities. The types of information we had access to included, but not limited to the following; prescriptions, diagnoses, medical history, surgical reports, smoking status, and physical examination.
2.2. Definitions
Drug-induced extrapyramidal symptoms (di-EPS) were defined as a diagnosis of dyskinesia, symptomatic dystonia or parkinsonism from the index date to 3 months after stopping the antipsychotic medication (APM) [9]. An updated list of drugs that may cause di-EPS was adopted [21].
2.3. Study Population
Outpatients who aged ≥18 years and initiated a single drug with the risk of extrapyramidal symptoms during the period 2010-2020 were included. Outpatients with any of the underlying conditions that may cause extrapyramidal symptoms were excluded. These conditions included dementia, hydrocephalus, subdural haemorrhage, hypoparathyroidism, neurodegeneration, and pantothenate kinase-associated neurodegeneration [22]. A total of 44,777 who took drugs with a risk of di-EPS were identified. The first prescription date of drugs that may induce extrapyramidal symptoms was defined as the index date. Among the identified samples, 6,091were excluded because they were diagnosed with one or more diseases before the index date. Moreover, 3,788 patients described having dyskinesia, parkinsonism, or dystonia before the index date were excluded. To evaluate the hazard ratio of di-EPS, a control group matched by gender, age, and duration of follow-up was included [case: control group = 1:2]. Control patients who did not fulfill the eligibility criteria mentioned before, were excluded from the study. During the study period, each patient was followed from the index date up to 36 months of taking the medication. Patients who were lost either because they died or no clinical data were available about them, were excluded.
2.4. Data Analysis
Data were analysed using the Statistical Package for Social Science (SPSS®) version 26 [IBM, Chicago, IL, US]. The analysis of the incidence of di-EPS included all patients who did not present with di-EPS at baseline. ANOVA and chi-square were used to compare the mean difference among groups for continuous and categorical variables, respectively. Cox proportional hazard regression was used to assess the factors associated with time to appearance di-EPS. Model reduction [stepwise]was performed to determine the effect of baseline covariates on the appearance of di-EPS. The baseline variables included were previously untreated patients, age at first contact, age, gender, marital status, smoking status, and comorbidity. A P value < 0.05 was considered statistically significant. A sensitivity analysis was performed to exclude patients who were already prescribed anticholinergic drugs, which can mask di-EPS.
3. RESULTS
A total of 34898 patients initiated treatment with a single drug with the risk of developing extrapyramidal symptoms at index date. The matched control group involved 69796 subjects. The age means of exposed and control participants were 46.6±14.9 and 47.4±16.1 years, respectively. Females accounted for 48.1% of exposed subjects. More than one-third, 34.9% of patients who initiated antipsychotic medications (APMs) were smokers. Approximately half of the exposed subjects, 48.3% had co-morbidities at index date; 22.1% and 24.8% of patients had diabetes mellitus and cardiovascular disease, respectively. Table 1 summarises the characteristics of patient groups.
Table 2 summarises the HRs extrapyramidal symptoms following exposure to drugs. Compared to patients on citalopram, patients taking paroxetine {HR: 8.6 [95%CI: 7.4-9.8], p=.0002}, imipramine {HR: 8.3, [7.1-10.5], p=0.01}, or fluoxetine {HR: 8.2 [95%CI: 6.8-9.3], p=.006}had a significantly higher risk of developing di-EPS. Risk factors for di-EPS development were smokers {HR: 1.7 [95%CI: 1.3-2.2], p=.02}, patients with tremor history {HR: 7.4 [95%CI: 5.9-8.3], p=.002}, and patients with history of taking antipsychotics {HR: 3.9 [95%CI: 2.5-4.6], p=0.001}.
| Item | Case | Control | P Value |
|---|---|---|---|
| Number | 34898 | 69796 | |
| Initial age (mean ±SD) | 46.6±14.9 | 47.4±16.1 | .004 |
| Gender | .49 | ||
| Male | 18116 (51.9%) | 35833 (51.3%) | |
| Female | 16782 (48.1%) | 33963 (48.7%) | |
| Marital Status | .07 | ||
| Single | 15949 (45.7%) | 32734 (46.9%) | |
| Married | 16204 (46.4%) | 31897 (45.7%) | |
| Divorced | 2745 (7.9%) | 5165 (7.4%) | |
| Smoking Status | .006 | ||
| Smoker | 12179 (34.9%) | 23172 (33.2%) | |
| Non-Smoker | 22719 (65.1%) | 46624 (66.8%) | |
| Comorbidity at index date | |||
| DM | 7503 (21.5%) | 15913 (22.8%) | .003 |
| CKD | 227 (0.7%) | 349 (0.5%) | .04 |
| CVD | 8478 (24.3%) | 16122 (23.1%) | .08 |
| Liver disorder | 294 (0.8%) | 768 (1.1%) | .04 |
| Tremor history | 147 (0.4%) | 244 (0.3%) | .12 |
| Other movement disorder | 196 (0.6%) | 351 (0.5%) | .13 |
| Medication used before index date | |||
| Antipsychotics | 516 (1.5%) | 1117 (1.6%) | .09 |
| Item | HR (95% CI) | P value |
|---|---|---|
| Gender (ref: male) | 1.00 | |
| Female | 2.6 (1.3-3.7) | .09 |
| Age (per 1 year) | 1.1 (0.8-1.2) | .003 |
| Smoking Status (ref: non-smokers) | 1.00 | |
| Smokers | 1.7 (1.3-2.2) | .02 |
| Comorbidity at index date (ref: without) | 1.00 | |
| DM | 1.2 (0.9-1.4) | .3 |
| CKD | 1.4 (1.1-1.6) | .5 |
| CVD | 1.6 (1.2-2.3) | .1 |
| Liver disorder | 0.7 (0.6-1.2) | .9 |
| Tremor history | 7.4 (5.9-8.3) | .002 |
| Other movement disorder | 3.2 (2.7-4.3) | .001 |
| Intervention (ref: Citalopram) | 1.00 | |
| Amitriptyline | 1.2 (0.8-3.7) | .001 |
| Clomipramine | 2.4 (1.7-4.3) | .0003 |
| Fluoxetine | 0.7 (0.4-1.3) | .006 |
| Fluvoxamine | 4.9 (3.7-5.9) | .0001 |
| Imipramine | 8.3 (7.1-10.5) | .001 |
| Nortriptyline | 4.1 (2.8-6.4) | .03 |
| Paroxetine | 8.6 (7.4-9.8) | .0002 |
| Medication used before index date ( ref: no antipsychotics) | 1.00 | |
| Antipsychotics | 3.9 (2.5-4.6) | .001 |
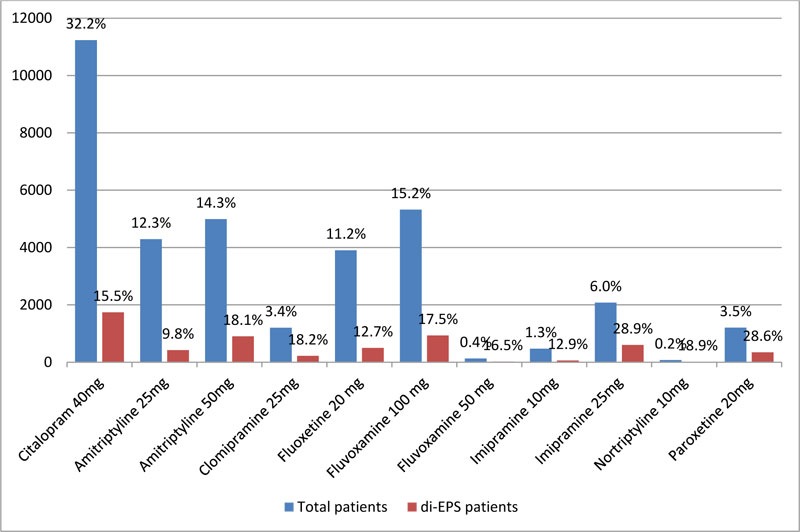
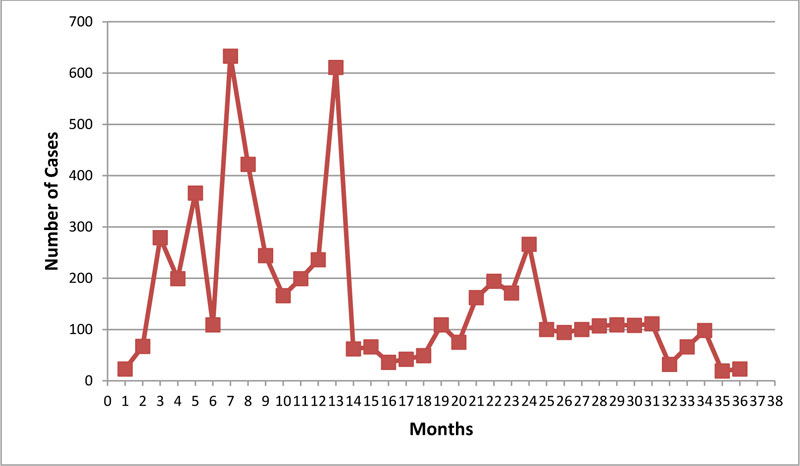
The overall incidence rate of drug-induced extrapyramidal symptoms (di-EPS) was 16.5%. As shown in Fig. (1), the most commonly prescribed APMs at index date were citalopram (32.2%), amitriptyline (26.6%), and fluvoxamine (15.6%). The incidence rate of di-EPS ranged between 9.8% and 28.9% depending on the drug initiated at index date; citalopram: 15.5%, amitriptyline 25mg:9.8%, amitriptyline 50mg: 18.1%, clomipramine: 18.2%, fluoxetine 20mg: 12.7%, fluvoxamine 100mg: 17.5%, fluvoxamine 50mg: 16.5%, imipramine 10mg: 12.9%, imipramine 25mg: 28.9%, nortriptyline 10mg: 18.9%, and paroxetine 20mg: 28.6%. Fig. (2) illustrates the number of di-EPS cases over time. High number of cases was diagnosed at 5, 7, and 14 months following the index date. Citalopram and amitriptyline were the most correlated with the high number of cases at 5,7 and 14 months.
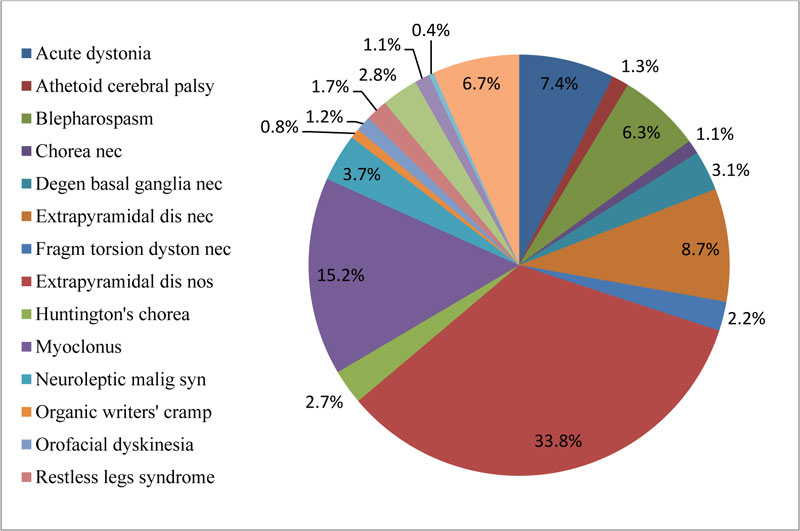
Our findings showed that 33.8% of di-EPS were unspecified; more than one-third of the patients, 35.6% suffered from myoclonus, blepharospasm, degenerative symp- toms of the basal ganglia, and organic writers' cramp. Fig. (3) illustrates di-EPS that patients suffered during the study.
4. DISCUSSION
The cumulative incidence of drug-induced extrapyramidal symptoms (di-EPS) ranged from 9.8% [Amitriptyline 25mg] to 28.9% [Imipramine 25mg]. Previously, Diego Novick et al. [23], conducted an observational study and found a similar incidence of di-EPS (7.7-32.8%). According to Shivanand B. Hiremath et al. [24], and Rissardo et al. [25], amitriptyline is associated with various movement disorders, including cervical dystonia, myoclonus, and dyskinesias. A case report indicated that concomitant use of amitriptyline and paroxetine could trigger dystonia development [26]. Our findings showed that the incidence of paroxetine-induced extrapyramidal symptoms is 28.6%. This is in line with previous studies that demonstrated the potential extrapyramidal symptoms of paroxetine [27].
We found that more than one-third of exposed subjects encountered symptoms such as myoclonus, blepharospasm, degenerative symptoms of the basal ganglia, and organic writers' cramp. Previous studies reported that tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs), particularly imipramine and paroxetine, could induce myoclonus [28-32]. The mechanisms of inducing these symptoms have not been completely elucidated.
A Chinese study indicated that APMs with high D2 receptor antagonism and disease duration are risk factors of EPS in patients with schizophrenia [33]. EPS were more frequently observed in male patients who were on risperidone [34]. The association between smoking and EPS has been controversial since many studies found no significant association between smoking and antipsychotics usage or risk of EPS [35]. However, others found that smoking schizophrenia patients had more severe positive symptoms but less severe EPS than non-smoking patients [36]
We found that age, smoking, tremor history, and history of taking antipsychotics before index date are risk factors for di-EPS development. There is substantial evidence that older individuals have a higher risk of di-EPS than younger patients [37]. The association between smoking and di-EPS has been controversial. Some studies linked smoking to the severity of symptoms; others were consistent with our findings and reported that smoking is a risk factor for di-EPS [38]. On the other hand, Jundong Jiang et al., reported no association between smoking and risk of di-EPS [35]. In addition, Matthew et al., found that smokers who received antidepressants did not have significantly more frequent or more severe extrapyramidal symptoms [39].
We found that a significant number of patients were diagnosed at 5, 7, and 14 months following the index date. We suspect that a typical and atypical antipsychotic drug have different modes of actions with different affinities, and efficacies. This might have contributed to our findings. While no decisive evidence has been found yet, we suggest further research in this area.
Due to the observational nature of this study and lack of randomisation, our findings are prone to selection bias and should be interpreted conservatively. In addition, our data source was a retrospective database, in which the approach for identifying extrapyramidal symptoms was indeterminate. However, Hakeem© database provided us with a specific date of prescription and medical history for patients involved. Our data were comprehensive since information on antipsychotic treatment in the 6 months before the index date was collected. Our major strength of this study is that we tracked prescriptions of drugs with the risk of extrapyramidal symptoms in outpatient clinics in Jordan for 10 years with a 3-year follow-up.
CONCLUSION
Initiating tricyclic antidepressants or serotonin reuptake inhibitors significantly increased the risk for extrapyramidal symptoms. Smoking, age, and history of taking antipsychotics were possible risk factors. Randomized controlled clinical trials are still necessary to produce high equality evidence.
AUTHOR’S CONTRIBUTION
All authors have contributed to all parts of the research, including the literature review, study design, study tool development, data analysis, and manuscript preparation and review.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ministry of Health, Jordan (Moh/REC/2019/177), and the Institutional Review Board (IRB) at Al-Balqa` Applied University, Jordan ( Approval. No. 6540).
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article is available from corresponding author [D.A.N] upon reasonable request.
FUNDING
No specific funding was received for this work
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We thank the Ministry of Health in Jordan for facilitating our research.


